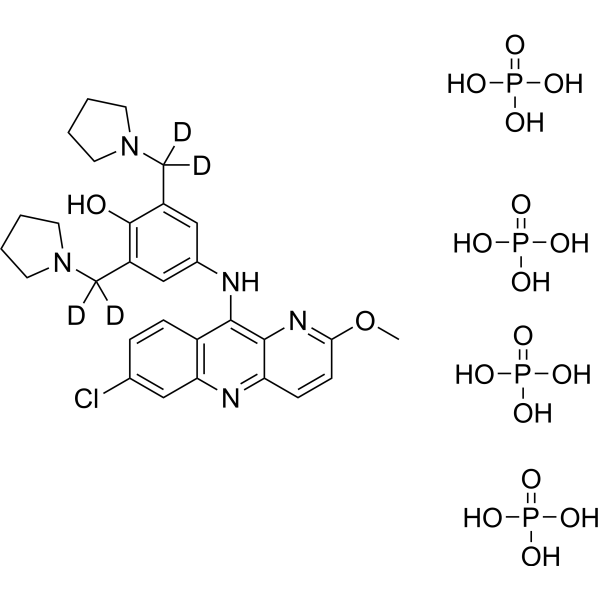
Download Files:
Pyronaridine-d4 (tetraphosphate)
SKU
HY-14749AS-Get quote
Category Isotope-Labeled Compounds
Tags Anti-infection, Infection, Parasite
Products Details
Product Description
– Pyronaridine-d4 (tetraphosphate) is the deuterium labeled Pyronaridine tetraphosphate[1]. Pyronaridine tetraphosphate is an orally active Mannich base anti-malarial agent. Pyronaridine tetraphosphate is active against P. falciparum and Echinococcus granulosus infection[2][3].
Web ID
– HY-14749AS
Shipping
– Room temperature
Applications
– COVID-19-immunoregulation
Molecular Formula
– C29H40D4ClN5O18P4
References
– [1]Russak EM, et al. Impact of Deuterium Substitution on the Pharmacokinetics of Pharmaceuticals. Ann Pharmacother. 2019 Feb;53(2):211-216.|[2]Vivas L, et al. Anti-malarial efficacy of pyronaridine and artesunate in combination in vitro and in vivo. Acta Trop. 2008 Mar;105(3):222-8.|[3]Jun Li, et al. Old drug repurposing for neglected disease: Pyronaridine as a promising candidate for the treatment of Echinococcus granulosus infections. EBioMedicine. 2020 Apr;54:102711.
CAS Number
– 1186026-25-4
Molecular Weight
– 914.06
SMILES
– O=P(O)(O)O.ClC1=CC2=C(C=C1)C(NC3=CC(C([2H])([2H])N4CCCC4)=C(C(C([2H])([2H])N5CCCC5)=C3)O)=C6C(C=CC(OC)=N6)=N2.O=P(O)(O)O.O=P(O)(O)O.O=P(O)(O)O
Clinical Information
– No Development Reported
Research Area
– Infection
Solubility
– 10 mM in DMSO
Target
– Parasite
Pathway
– Anti-infection
Product type
– Isotope-Labeled Compounds
Disclaimer: All products are for Research use only unless clearly stated otherwise on the product datasheet. Datasheets provided on the website are drafts for reference purpose only and you are requested to always refer to the hard copy included in the kit for your experimentation. Agdia Products are available for delivery only in Canada.