Download Files:
PT2399
SKU
HY-108697-10 mg
Category Reference compound
Tags Cancer, HIF/HIF Prolyl-Hydroxylase, Metabolic Enzyme/Protease
$370 – $2,400
Products Details
Product Description
– PT2399 is a potent and selective HIF-2α antagonist, which directly binds to HIF-2α PAS B domain with an IC50 of 6 nM. PT2399 displays potent antitumor activity in vivo[1][2][3].
Web ID
– HY-108697
Storage Temperature
– -20°C (Powder, stored under nitrogen)
Shipping
– Blue Ice
Applications
– Cancer-programmed cell death
Molecular Formula
– C17H10F5NO4S
References
– [1]Wehn PM, et al. Design and Activity of Specific Hypoxia-Inducible Factor-2α (HIF-2α) Inhibitors for the Treatment of Clear Cell Renal Cell Carcinoma: Discovery of Clinical Candidate (S)-3-((2,2-Difluoro-1-hydroxy-7-(methylsulfonyl)-2,3-dihydro-1 H-inden-4-yl)oxy)-5-fluorobenzonitrile (PT2385). J Med Chem. 2018 Nov 8;61(21):9691-9721.|[2]Chen W, et al. Targeting renal cell carcinoma with a HIF-2 antagonist. Nature. 2016 Nov 3;539(7627):112-117.|[3]Cho H, et al. On-Target Efficacy of a HIF2α Antagonist in Preclinical Kidney Cancer Models. Nature. Nature. 2016 Nov 3;539(7627):107-111.
CAS Number
– 1672662-14-4
Molecular Weight
– 419.32
Compound Purity
– 99.93
SMILES
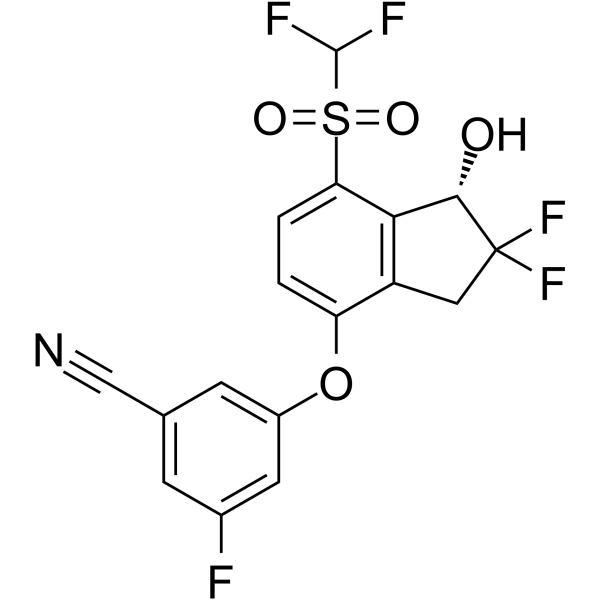
– N#CC1=CC(F)=CC(OC2=CC=C(S(=O)(C(F)F)=O)C3=C2CC(F)(F)[C@H]3O)=C1
Clinical Information
– No Development Reported
Research Area
– Cancer
Solubility
– DMSO : ≥ 200 mg/mL
Target
– HIF/HIF Prolyl-Hydroxylase
Pathway
– Metabolic Enzyme/Protease
Product type
– Reference compound
Disclaimer: All products are for Research use only unless clearly stated otherwise on the product datasheet. Datasheets provided on the website are drafts for reference purpose only and you are requested to always refer to the hard copy included in the kit for your experimentation. Agdia Products are available for delivery only in Canada.






