Download Files:
PSI-7409
Products Details
Product Description
– PSI-7409 is the active 5′-triphosphate metabolite of Sofosbuvir (PSI-7977). Sofosbuvir (PSI-7977) is a selective and highly active nucleotide analog inhibitor of HCV.
Web ID
– HY-15745
Storage Temperature
– -20°C, 3 years (Powder)
Shipping
– Blue Ice
Applications
– Cancer-programmed cell death
Molecular Formula
– C10H16FN2O14P3
Citations
– Antiviral Res. 2020 Mar;175:104708.|Biochem Biophys Res Commun. 2022 Dec 8;641:50-56.|Cell. 2022 Nov 10;185(23):4347-4360.e17.|J Virol Methods. 2021 Sep 14;298:114283.|Microbiol Spectr. 2022 Aug 18;e0272922.|Asian J Pharm Sci. 21 October 2021.
References
– [1]Lam AM, et al. PSI-7851, a pronucleotide of beta-D-2′-deoxy-2′-fluoro-2′-C-methyluridine monophosphate, is a potent and pan-genotype inhibitor of hepatitis C virus replication. Antimicrob Agents Chemother. 2010 Aug;54(8):3187-96.|[2]Murakami E, et al. Mechanism of activation of PSI-7851 and its diastereoisomer PSI-7977.
CAS Number
– 1015073-42-3
Molecular Weight
– 500.16
Compound Purity
– 98.03
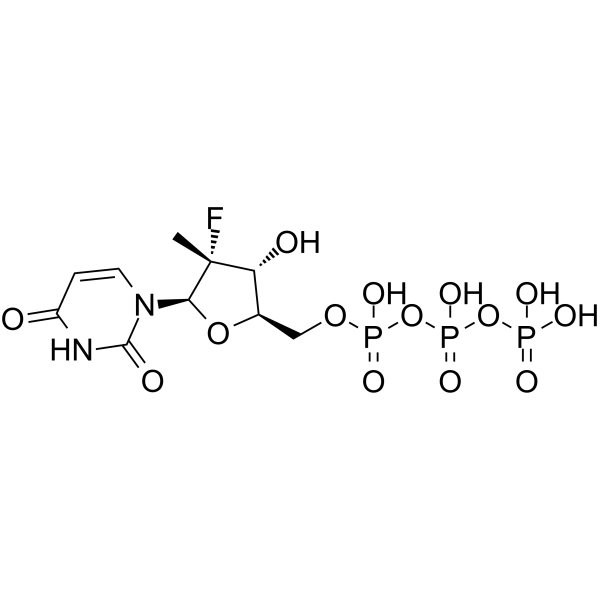
SMILES
– O[C@@H]([C@@](C)(F)[C@H](N1C(NC(C=C1)=O)=O)O2)[C@H]2COP(O)(OP(OP(O)(O)=O)(O)=O)=O
Clinical Information
– No Development Reported
Research Area
– Cancer
Solubility
– DMF : 10 mg/mL (ultrasonic;warming;heat to 80°C)|DMSO : 1 mg/mL (ultrasonic)|H2O : 50 mg/mL (ultrasonic)
Target
– HCV
Pathway
– Anti-infection
Product type
– Reference compound
Disclaimer: All products are for Research use only unless clearly stated otherwise on the product datasheet. Datasheets provided on the website are drafts for reference purpose only and you are requested to always refer to the hard copy included in the kit for your experimentation. Agdia Products are available for delivery only in Canada.





