Download Files:
Ponalrestat
SKU
HY-106697-10 mg
Category Reference compound
Tags Aldose Reductase, Metabolic Disease, Metabolic Enzyme/Protease
$32 – $185
Products Details
Product Description
– Ponalrestat (ICI 128436) is an orally active, selective and noncompetitive aldose reductase (AKR1B1; ALR) inhibitor. Ponalrestat selectively inhibits ALR2 (Ki=7.7 nM) over ALR1 (Ki=60 μM). Ponalrestat inhibits the conversion of glucose to sorbitol[1][2][3].
Web ID
– HY-106697
Storage Temperature
– 4°C (Powder, sealed storage, away from moisture)
Shipping
– Room Temperature
Applications
– Metabolism-sugar/lipid metabolism
Molecular Formula
– C17H12BrFN2O3
References
– [1]Ward WH, et al. Ponalrestat: a potent and specific inhibitor of aldose reductase. Biochem Pharmacol. 1990 Jan 15;39(2):337-46.|[2]Bresson E, et al. The human aldose reductase AKR1B1 qualifies as the primary prostaglandin F synthase in the endometrium. J Clin Endocrinol Metab. 2011 Jan;96(1):210-9.|[3]Calcutt NA, et al. Prevention of sensory disorders in diabetic Sprague-Dawley rats by aldose reductase inhibition or treatment with ciliary neurotrophic factor. Diabetologia. 2004 Apr;47(4):718-24.
CAS Number
– 72702-95-5
Molecular Weight
– 391.19
Compound Purity
– 99.71
SMILES
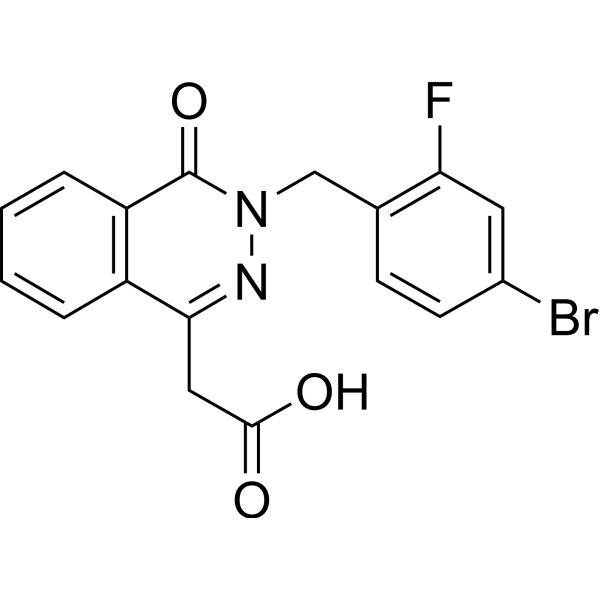
– OC(CC(C1=C2C=CC=C1)=NN(CC3=C(C=C(Br)C=C3)F)C2=O)=O
Clinical Information
– No Development Reported
Research Area
– Metabolic Disease
Solubility
– DMSO : 62.5 mg/mL (ultrasonic)
Target
– Aldose Reductase
Pathway
– Metabolic Enzyme/Protease
Product type
– Reference compound
Disclaimer: All products are for research use only unless clearly stated otherwise on the product datasheet. Datasheets provided on the website are drafts for reference purpose only and you are requested to always refer to the hard copy included in the kit for your experimentation.





