Download Files:
Plerixafor
SKU
HY-10046-10 mg
Category Reference compound
Tags Anti-infection;GPCR/G Protein;Immunology/Inflammation, Cancer; Infection; Endocrinology; Inflammation/Immunology, CXCR;HIV
$54 – $417
Products Details
Product Description
– Plerixafor (AMD 3100) is a selective CXCR4 antagonist with an IC50 of 44 nM. Plerixafor, an immunostimulant and a hematopoietic stem cell (HSC) mobilizer, is an allosteric agonist of CXCR7. Plerixafor inhibits HIV-1 and HIV-2 replication with an EC50 of 1-10 nM[1][2][3][4][7].
Web ID
– HY-10046
Storage Temperature
– -20°C, 3 years; 4°C, 2 years (Powder)
Shipping
– Room Temperature
Applications
– COVID-19-anti-virus
Molecular Formula
– C28H54N8
Citations
– ACS Infect Dis. 2023 Oct 5.|Andrology. 2022 Sep 16.|Bioact Mater. 2021 Jan 7;6(7):2039-2057.|Biochem Biophys Res Commun. 2021 Jan 1;534:337-342.|Biomed Pharmacother. 2020, 110610.|BMC Complement Altern Med. 2018 Dec 12;18(1):330. |Br J Haematol. 2022 Dec 19.|Cancer Lett. 2022 Oct 7;551:215944.|Cell Death Dis. 2017 Jan 19;8(1):e2560. |Cell Death Dis. 2022 Feb 4;13(2):118.|Cell Death Dis. 2023 Mar 28;14(3):219.|Cell Mol Immunol. 2020 Mar;17(3):283-299.|Cell Physiol Biochem. 2018;46(3):890-906. |Cell Signal. 2020 Feb;66:109488.|Cell Tissue Res. 2020 Jun;380(3):469-486.|Cell Transplant. 2022 Jan-Dec;31:9636897221129171.|Cells. 2019 Jul 22;8(7):761.|Cells. 2022 Jan 4;11(1):155.|Chinese Journal of Tissue Engineering Research. 2014,18(45): 7327-7332.|Drug Des Devel Ther. 2022 Jan 6;16:67-81.|Exp Ther Med. July 19, 2021.|Fertil Steril. 2020 May;113(5):1067-1079.e5.|Int J Biol Sci. 2017 May 5;13(5):604-614.|Int J Mol Sci. 2023 Aug 13, 24(16), 12740.|Int J Nanomedicine. 2023 Jul 31;18:4329-4346.|J Diabetes Complicat. 2020 Oct;34(10):107654.|J Mater Chem B. 2018 Apr 7;6(13):1951-1964. |J Oncol. 08 Oct 2021.|J Steroid Biochem Mol Biol. 2021 Jun 3;105926.|J Transl Med. 2023 Sep 5;21(1):593.|Kaohsiung J Med Sci. 2021 Nov 6.|Ludwig maxime clinic. University of Munich. 2019 Apr.|Mol Med Rep. 2020 Oct;22(4):3201-3212.|Nano Today. 2022, 47: 101689.|Oncogene. 2019 Jun;38(25):5021-5037. |Oncol Lett. 2018 Sep;16(3):3976-3982. |Patent. US20200360478A1.|PLoS One. 2021 Mar 1;16(3):e0247707.|Research Square Preprint. 2021 Aug.|Research Square Preprint. 2021 Sep.|Research Square Preprint. 2022 Jul.|Research Square Preprint. 2023 Oct 23.|SSRN. 5 Feb 2022.|Stem Cells Int. 2020 Jul 7;2020:1498315.|Theranostics. 2021 Jan 1;11(6):2612-2633.|Transl Stroke Res. 2021 Jun 25.|Universität Hamburg. Informatics and Natural Sciences. 2021 Mar 19.|Uppsala University. Department of Pharmaceutical Biosciences. 2022 Feb.|Adv Funct Mater. 2020, 2000309.|Anticancer Drugs. 2017 Oct;28(9):935-942.|Brain Behav Immun. 2017 Jan;59:322-332.|Mol Ther-Nucl Acids. 2023 Mar 9.|Oncogene. 2022 Oct;41(41):4633-4644.|Oxid Med Cell Longev. 2021 Aug 2;2021:9993240.|Patent. US20190133998Al|Pharmaceutics. 2021 Mar 24;13(4):439.|Ruprecht-Karls-University Heidelberg. 2023 Aug 3.
References
– [1]De Clercq E, et al. Mozobil® (Plerixafor, AMD3100), 10 years after its approval by the US Food and Drug Administration. Antivir Chem Chemother. 2019 Jan-Dec;27:2040206619829382.|[2]Seki JT, et al. Chemical Stability of Plerixafor after Opening of Single-Use Vial. Can J Hosp Pharm. 2017 Jul-Aug;70(4):270-275.|[3]Schols D, et al. HIV co-receptor inhibitors as novel class of anti-HIV drugs. Antiviral Res. 2006 Sep;71(2-3):216-26.|[4]Yang J, et al. Continuous AMD3100 Treatment Worsens Renal Fibrosis through Regulation of Bone Marrow Derived Pro-Angiogenic Cells Homing and T-Cell-Related Inflammation. PLoS One. 2016 Feb 22;11(2):e0149926.|[5]Chu PY, et al. CXCR4 Antagonism Attenuates the Development of Diabetic Cardiac Fibrosis. PLoS One. 2015 Jul 27;10(7):e0133616.|[6]Mercurio L, et al. Targeting CXCR4 by a selective peptide antagonist modulates tumor microenvironment and microglia reactivity in a human glioblastoma model. J Exp Clin Cancer Res. 2016 Mar 25;35:55.|[7]Zabel BA, et al. Elucidation of CXCR7-mediated signaling events and inhibition of CXCR4-mediated tumor cell transendothelial migration by CXCR7 ligands. J Immunol. 2009 Sep 1;183(5):3204-11.|[8]Zheng J, et al. Toward Normalization of the Tumor Microenvironment for Cancer Therapy. Integr Cancer Ther. 2019;18:1534735419862352.
CAS Number
– 110078-46-1
Molecular Weight
– 502.78
Compound Purity
– 98.61
SMILES
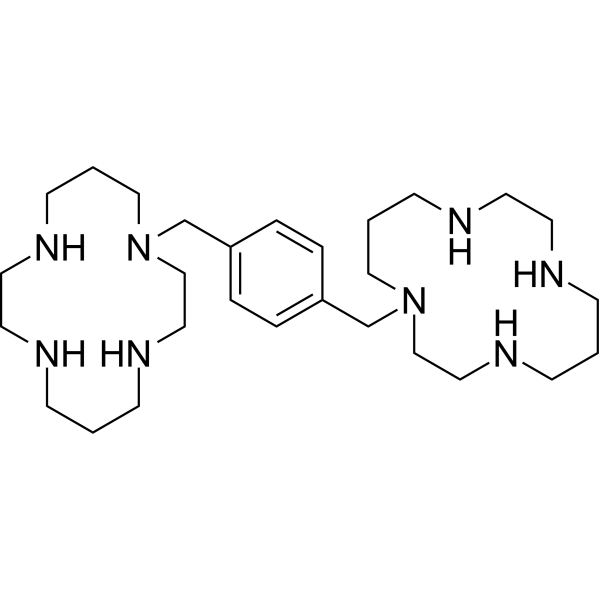
– C1(CN2CCCNCCNCCCNCC2)=CC=C(C=C1)CN3CCNCCCNCCNCCC3
Clinical Information
– Launched
Research Area
– Cancer; Infection; Endocrinology; Inflammation/Immunology
Solubility
– DMF : 1 mg/mL (ultrasonic)|DMSO : 1.96 mg/mL (ultrasonic;warming;adjust pH to 5 with HCl;heat to 60°C)|Ethanol : 50 mg/mL (ultrasonic)|H2O : < 0.1 mg/mL (ultrasonic)
Target
– CXCR;HIV
Isoform
– CXCR4;CXCR7;HIV-1;HIV-2
Pathway
– Anti-infection;GPCR/G Protein;Immunology/Inflammation
Product type
– Reference compound
Disclaimer: All products are for Research use only unless clearly stated otherwise on the product datasheet. Datasheets provided on the website are drafts for reference purpose only and you are requested to always refer to the hard copy included in the kit for your experimentation. Agdia Products are available for delivery only in Canada.






