Download Files:
Pirmenol
SKU
HY-100795-Get quote
Category Reference compound
Tags Cardiovascular Disease, GPCR/G Protein;Membrane Transporter/Ion Channel;Neuronal Signaling, mAChR;Potassium Channel
Products Details
Product Description
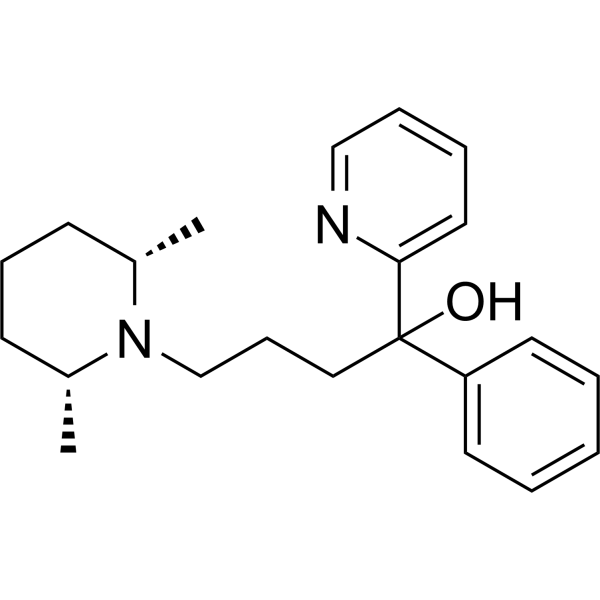
– Pirmenol is an orally active antiarrhythmic agent. Pirmenol inhibits IK.ACh (IC50: 0.1 μM) by blocking mAchR. Pirmenol can be used in the research of cardiovascular disease, such as atrial fibrillation[1][2][4].
Web ID
– HY-100795
Shipping
– Room temperature
Applications
– Neuroscience-Neuromodulation
Molecular Formula
– C22H30N2O
References
– [1]Watanabe Y, et al. Pirmenol inhibits muscarinic acetylcholine receptor-operated K+ current in the guinea pig heart. Eur J Pharmacol. 1997 Oct 29;338(1):71-4.|[2]Schardein JL, et al. Preclinical toxicology studies with a new antiarrhythmic agent: Pirmenol hydrochloride (CI-845). Toxicol Appl Pharmacol. 1980 Nov;56(2):294-301.|[3]T Sawanobori, et al. Electrophysiologic and antiarrhythmic actions of pirmenol on rabbit and guinea pig cardiac preparations. J Cardiovasc Pharmacol. 1990 Dec;16(6):975-83. |[4]T E Mertz, et al. Pirmenol hydrochloride (CI-845): antiarrhythmic profile in coronary artery ligated conscious dogs. J Cardiovasc Pharmacol. 1980 Sep-Oct;2(5):527-41.
CAS Number
– 68252-19-7
Molecular Weight
– 338.49
SMILES
– OC(C1=CC=CC=C1)(CCCN2[C@@H](C)CCC[C@H]2C)C3=CC=CC=N3
Clinical Information
– Launched
Research Area
– Cardiovascular Disease
Solubility
– 10 mM in DMSO
Target
– mAChR;Potassium Channel
Pathway
– GPCR/G Protein;Membrane Transporter/Ion Channel;Neuronal Signaling
Product type
– Reference compound
Disclaimer: All products are for Research use only unless clearly stated otherwise on the product datasheet. Datasheets provided on the website are drafts for reference purpose only and you are requested to always refer to the hard copy included in the kit for your experimentation. Agdia Products are available for delivery only in Canada.






