Download Files:
Pirarubicin
SKU
HY-13725-10 mg
Category Reference compound
Tags Anti-infection;Autophagy;Cell Cycle/DNA Damage, Antibiotic;Autophagy;Bacterial;Topoisomerase, Cancer; Infection
$42 – $418
Products Details
Product Description
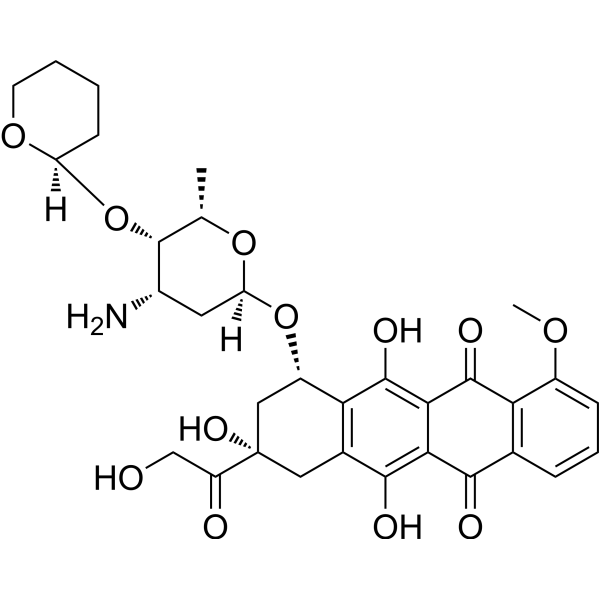
– Pirarubicin is an anthracycline antibiotics, acts as a topoisomerase II inhibitor, and is a widely used for treatment of various cancers, in particular, solid tumors.
Web ID
– HY-13725
Storage Temperature
– 4°C (Powder, protect from light)
Shipping
– Room Temperature
Applications
– Cancer-programmed cell death
Molecular Formula
– C32H37NO12
Citations
– Cancers (Basel). 2023 Apr 26, 15(9), 2487.|E3S Web of Conferences. 233, 02010 (2021).|Genomics. 2021 Nov 26;S0888-7543(21)00410-9.|Int J Mol Med. 2023 May 17.|J Mol Med (Berl). 2019 Aug;97(8):1183-1193.|Toxicol Appl Pharmacol. 2023 Feb 3;462:116411.|Cancer Drug Resist. 2020 Sep 12. |Heliyon. June 2022, e09643.|Int J Pharm. 2022 May 25;620:121761.|J Mol Liq. 2020, 114633.|J Mol Liq. 2023 Jun 12, 122324.
References
– [1]Takigawa N, et al. Cytotoxic effect of topoisomerase II inhibitors against adriamycin- and etoposide-resistant small cell lung cancer sublines. Gan To Kagaku Ryoho. 1993 May;20(7):929-35.|[2]Nagai K, et al. Relationships between the in vitro cytotoxicity and transport characteristics of pirarubicin and doxorubicin in M5076 ovarian sarcoma cells, and comparison with those in Ehrlich ascites carcinoma cells. Cancer Chemother Pharmacol. 2002 Mar;49(3):244-50. Epub 2002 Jan 8.|[3]Li K, et al. Pirarubicin induces an autophagic cytoprotective response through suppression of the mammalian target of rapamycin signaling pathway in human bladder cancer cells. Biochem Biophys Res Commun. 2015 May 1;460(2):380-5.|[4]Wang YD, et al. Cardioprotective effects of rutin in rats exposed to pirarubicin toxicity. J Asian Nat Prod Res. 2017 Oct 27:1-13.
CAS Number
– 72496-41-4
Molecular Weight
– 627.64
Compound Purity
– 99.61
SMILES
– O=C1C2=C(C=CC=C2OC)C(C3=C(O)C4=C([C@@H](O[C@@]5([H])C[C@H](N)[C@H](O[C@@]6([H])OCCCC6)[C@H](C)O5)C[C@@](C(CO)=O)(O)C4)C(O)=C31)=O
Clinical Information
– Launched
Research Area
– Cancer; Infection
Solubility
– DMSO : 10 mg/mL (ultrasonic;warming;heat to 80°C)
Target
– Antibiotic;Autophagy;Bacterial;Topoisomerase
Isoform
– Topo II
Pathway
– Anti-infection;Autophagy;Cell Cycle/DNA Damage
Product type
– Reference compound
Disclaimer: All products are for Research use only unless clearly stated otherwise on the product datasheet. Datasheets provided on the website are drafts for reference purpose only and you are requested to always refer to the hard copy included in the kit for your experimentation. Agdia Products are available for delivery only in Canada.






