Download Files:
Phensuximide
$70 – $620
Products Details
Product Description
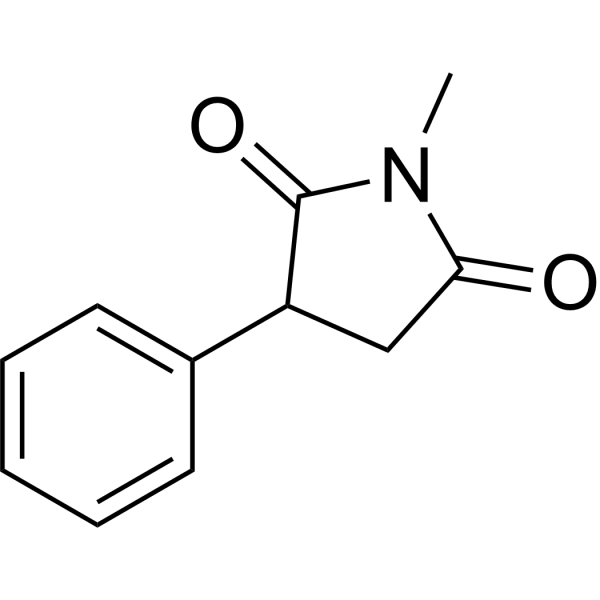
– Phensuximide is an orally active succinimide antiepileptic and anticonvulsant agent. Phensuximide inhibits cyclic AMP and cyclic GMP accumulation in depolarized brain tissue. Phensuximide can be used for the study of seizure and petit mal[1][3].
Web ID
– HY-B1730
Storage Temperature
– -20°C, 3 years; 4°C, 2 years (Powder)
Shipping
– Room Temperature
Applications
– Metabolism-protein/nucleotide metabolism
Molecular Formula
– C11H11NO2
References
– [1]S D Hall,et al. Characterization and inhibition of mephenytoin 4-hydroxylase activity in human liver microsomes. The JOURNAL OP PHARMAcOLOGY AND EXPERIMENTAL THERAPEUTICS|[2]J G MILLICHAP, et al. Milontin: A New Drug in the Treatment of Petit Mal.Lancet. 1952 Nov 8;2(6741):907-10.|[3]G O Rankin, et al. Urinary Tract Effects of Phensuximide in the Sprague-Dawley and Fischer 344 Rat. J Appl Toxicol. . 1986 Oct;6(5):349-56.|[4]J A Ferrendelli, et al. Inhibitory Effects of Anticonvulsant Drugs on Cyclic Nucleotide Accumulation in Brain. Ann Neurol. 1979 Jun;5(6):533-8.
CAS Number
– 86-34-0
Molecular Weight
– 189.21
Compound Purity
– 99.35
SMILES
– O=C(C(C1=CC=CC=C1)C2)N(C)C2=O
Clinical Information
– Launched
Research Area
– Metabolic Disease; Neurological Disease
Solubility
– DMSO : 250 mg/mL (ultrasonic)
Target
– Others
Pathway
– Others
Product type
– Reference compound
Disclaimer: All products are for Research use only unless clearly stated otherwise on the product datasheet. Datasheets provided on the website are drafts for reference purpose only and you are requested to always refer to the hard copy included in the kit for your experimentation. Agdia Products are available for delivery only in Canada.






