Download Files:
PF-06291874
$70 – $680
Products Details
Product Description
– PF-06291874 is a highly potent, non-peptide and orally active glucagon receptor antagonist. PF-06291874 is under the study for type 2 diabetes mellitus (T2DM)[1][2].
Web ID
– HY-19947
Storage Temperature
– -20°C, 3 years; 4°C, 2 years (Powder)
Shipping
– Room Temperature
Applications
– Metabolism-sugar/lipid metabolism
Molecular Formula
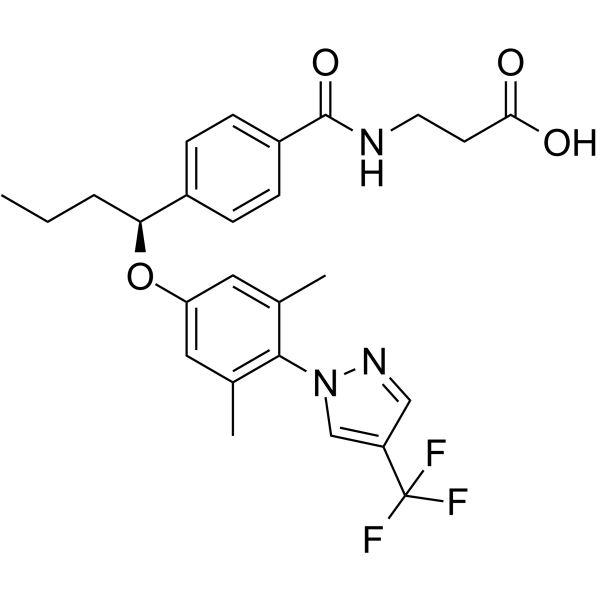
– C26H28F3N3O4
References
– [1]Derek J Nunez, et al. Glucagon receptor as a drug target: A witches’ brew of eye of newt (peptides) and toe of frog (receptors). Diabetes Obes Metab. 2018 Feb;20(2):233-237.|[2]Esther C.Y. Lee et al. Identification of a novel conformationally constrained glucagon receptor antagonist. Bioorg Med Chem Lett, 2014 Feb 1, 24(3):839-44.|[3]D J Kazierad, et al. Effects of multiple ascending doses of the glucagon receptor antagonist PF-06291874 in patients with type 2 diabetes mellitus. Diabetes Obes Metab. 2016 Aug;18(8):795-802.
CAS Number
– 1393124-08-7
Molecular Weight
– 503.51
Compound Purity
– 99.18
SMILES
– CCC[C@H](OC1=CC(C)=C(N2C=C(C(F)(F)F)C=N2)C(C)=C1)C3=CC=C(C(NCCC(O)=O)=O)C=C3
Clinical Information
– Phase 2
Research Area
– Metabolic Disease
Solubility
– DMSO : 100 mg/mL (ultrasonic)
Target
– GCGR
Pathway
– GPCR/G Protein
Product type
– Reference compound
Disclaimer: All products are for Research use only unless clearly stated otherwise on the product datasheet. Datasheets provided on the website are drafts for reference purpose only and you are requested to always refer to the hard copy included in the kit for your experimentation. Agdia Products are available for delivery only in Canada.







