Download Files:
Perindopril (erbumine)
SKU
HY-B0130A-100 mg
Category Reference compound
Tags Angiotensin-converting Enzyme (ACE);Apoptosis, Apoptosis;Metabolic Enzyme/Protease, Cardiovascular Disease; Cancer
$72 – $216
Products Details
Product Description
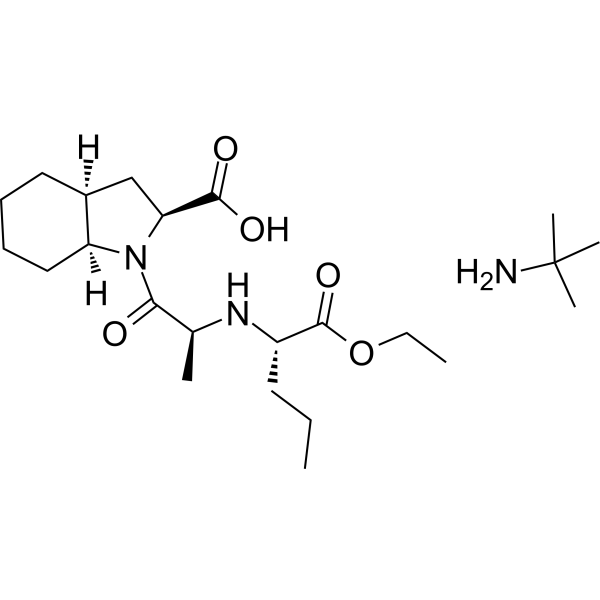
– Perindopril erbumine (Perindopril tert-butylamine salt) is a long-acting angiotensin-converting enzyme (ACE) inhibitor.
Web ID
– HY-B0130A
Storage Temperature
– 4°C (Powder, sealed storage, away from moisture)
Shipping
– Room Temperature
Applications
– COVID-19-anti-virus
Molecular Formula
– C23H43N3O5
Citations
– Biosci Rep. 2021 Oct 29;41(10):BSR20211598.|Br J Pharmacol. 2021 Mar;178(5):1164-1181.|Evid-Based Compl Alt. 2022 May 21;2022:5812105.|Biomed Pharmacother. 2023 Feb 6;160:114370.
References
– [1]Vaclavik J, Perindopril in the treatment of hypertension and cardiovascular diseases: evolution continues with the orodispersible dosage form. Vnitr Lek. 2013 Apr;59(4):290-4.|[2]Napalkov DA. Therapy with perindopril: organoprotection, not just the antihypertensive effect. Kardiologiia. 2012;52(12):80-3.
CAS Number
– 107133-36-8
Molecular Weight
– 441.60
Compound Purity
– 99.98
SMILES
– O=C([C@H]1N(C([C@@H](N[C@H](C(OCC)=O)CCC)C)=O)[C@@]2([H])CCCC[C@@]2([H])C1)O.CC(C)(C)N
Clinical Information
– Launched
Research Area
– Cardiovascular Disease; Cancer
Solubility
– DMSO : 10 mg/mL (ultrasonic)|H2O : ≥ 50 mg/mL
Target
– Angiotensin-converting Enzyme (ACE);Apoptosis
Pathway
– Apoptosis;Metabolic Enzyme/Protease
Product type
– Reference compound
Disclaimer: All products are for Research use only unless clearly stated otherwise on the product datasheet. Datasheets provided on the website are drafts for reference purpose only and you are requested to always refer to the hard copy included in the kit for your experimentation. Agdia Products are available for delivery only in Canada.






