Download Files:
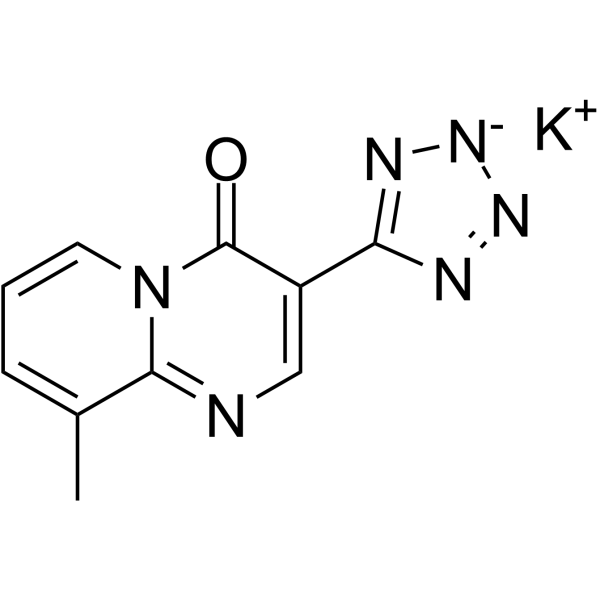
Pemirolast (potassium)
SKU
HY-B0538A-10 mg
Category Reference compound
Tags GPCR/G Protein;Immunology/Inflammation;Neuronal Signaling, Histamine Receptor, Inflammation/Immunology; Endocrinology
$39 – $275
Web ID
– HY-B0538A
Storage Temperature
– 4°C (Powder, sealed storage, away from moisture)
Shipping
– Room Temperature
Applications
– COVID-19-immunoregulation
Molecular Formula
– C10H7KN6O
References
– [1]Kemp, J.P., et al., Pemirolast, a new oral nonbronchodilator drug for chronic asthma. Ann Allergy, 1992. 68(6): p. 488-91.|[2]Tatsushima, Y., et al., Pemirolast reduces cisplatin-induced kaolin intake in rats. Eur J Pharmacol, 2011. 661(1-3): p. 57-62.|[3]Itoh, Y., et al., Pemirolast potently attenuates paclitaxel hypersensitivity reactions through inhibition of the release of sensory neuropeptides in rats. Neuropharmacology, 2004. 46(6): p. 888-94.|[4]Abelson, M.B., et al., Pemirolast potassium 0.1% ophthalmic solution is an effective treatment for allergic conjunctivitis: a pooled analysis of two prospective, randomized, double-masked, placebo-controlled, phase III studies. J Ocul Pharmacol Ther, 2002. 18(5): p. 475-88.
CAS Number
– 100299-08-9
Molecular Weight
– 266.30
Compound Purity
– 99.93
SMILES
– O=C1C(C2=N[N-]N=N2)=CN=C3N1C=CC=C3C.[K+]
Clinical Information
– Launched
Research Area
– Inflammation/Immunology; Endocrinology
Solubility
– H2O : 50 mg/mL (ultrasonic)
Target
– Histamine Receptor
Isoform
– H1 Receptor
Pathway
– GPCR/G Protein;Immunology/Inflammation;Neuronal Signaling
Product type
– Reference compound
Disclaimer: All products are for Research use only unless clearly stated otherwise on the product datasheet. Datasheets provided on the website are drafts for reference purpose only and you are requested to always refer to the hard copy included in the kit for your experimentation. Agdia Products are available for delivery only in Canada.






