Download Files:
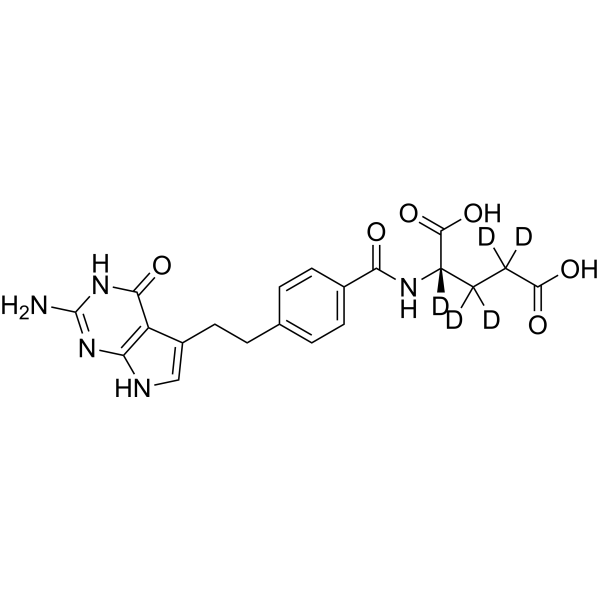
Pemetrexed-d5
SKU
HY-10820S-Get quote
Category Isotope-Labeled Compounds
Tags Antifolate;Autophagy, Autophagy;Cell Cycle/DNA Damage, Cancer
Products Details
Product Description
– Pemetrexed-d5 is the deuterium labeled Pemetrexed[1]. Pemetrexed (LY231514) is an antifolate, the Ki values of the pentaglutamate of Pemetrexed (LY231514) are 1.3, 7.2, and 65 nM for inhibits thymidylate synthase (TS), dihydrofolate reductase (DHFR), and glycinamide ribonucleotide formyltransferase (GARFT), respectively[2].
Web ID
– HY-10820S
Shipping
– Room temperature
Applications
– Cancer-programmed cell death
Molecular Formula
– C20H16D5N5O6
References
– [1]Russak EM, et al. Impact of Deuterium Substitution on the Pharmacokinetics of Pharmaceuticals. Ann Pharmacother. 2019 Feb;53(2):211-216.|[2]Shih C, et al. LY231514, a pyrrolo[2,3-d]pyrimidine-based antifolate that inhibits multiple folate-requiring enzymes. Cancer Res. 1997 Mar 15;57(6):1116-23.|[3]Anraku M, et al. Synergistic antitumor effects of regulatory T cell blockade combined with pemetrexed in murine malignantmesothelioma. J Immunol. 2010 Jul 15;185(2):956-66.
CAS Number
– 1129408-57-6
Molecular Weight
– 432.44
SMILES
– O=C(O)C([2H])([2H])C([2H])([2H])[C@](C(O)=O)([2H])NC(C1=CC=C(CCC2=CNC(N=C(N)N3)=C2C3=O)C=C1)=O
Clinical Information
– No Development Reported
Research Area
– Cancer
Solubility
– 10 mM in DMSO
Target
– Antifolate;Autophagy
Pathway
– Autophagy;Cell Cycle/DNA Damage
Product type
– Isotope-Labeled Compounds
Disclaimer: All products are for Research use only unless clearly stated otherwise on the product datasheet. Datasheets provided on the website are drafts for reference purpose only and you are requested to always refer to the hard copy included in the kit for your experimentation. Agdia Products are available for delivery only in Canada.