Download Files:
Oseltamivir (phosphate)
$60 – $102
Products Details
Product Description
– Oseltamivir phosphate (GS 4104) is a neuraminidase inhibitor recommended for the treatment and prophylaxis of influenza A and B.
Web ID
– HY-17016
Storage Temperature
– 4°C (Powder, sealed storage, away from moisture)
Shipping
– Room Temperature
Applications
– COVID-19-anti-virus
Molecular Formula
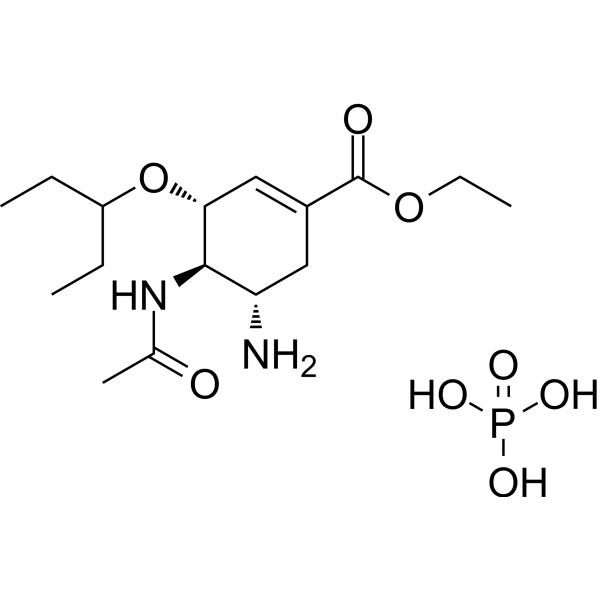
– C16H31N2O8P
Citations
– Antiviral Res. 2020 Apr;176:104751. |Arch Pharm Res. 2018 Jun;41(6):664-676.|Bioeng Transl Med. 2020 Dec 1;6(1):e10196.|Biomed Pharmacother. 2018 Jan;97:385-394. |bioRxiv. 2023 Aug 12.|Biosci Biotechnol Biochem. 2020 Apr;84(4):774-779.|Cell Prolif. 2021 Jan;54(1):e12953.|Chemosphere. 2015 Jul;131:41-7.|Front Cell Dev Biol. 17 December 2021.|Fundação Oswaldo Cruz. Instituto Oswaldo Cruz. Laboratório de Transmissores de Hematozoários. 2020 Sep.|Inflammation. 2023 Aug 21.|Int J Mol Sci. 2019 Dec 12;20(24):6261.|Int J Mol Sci. 2022 Apr 1;23(7):3940.|J Ethnopharmacol. 2023 Oct 6:117290.|J Med Virol. 2023 Jul;95(7):e28968.|Lab Invest. 2020 Dec;100(12):1602-1617.|Sci Rep. 2021 Aug 11;11(1):16293.|Signal Transduct Target Ther. 2021 Apr 24;6(1):165.|Virology. 2020 Jun;545:1-9.|Viruses. 2018 Jun 13;10(6). pii: E325. |Antiviral Res. 2016 Mar;127:68-78. |Food Funct. 2016 Apr 20;7(4):2052-9. |Phytochemistry. 2023 Aug 17;113829.|Virology. 2023 Dec 2, 109954.
References
– [1]Huang H, et al. Transplacental transfer of Oseltamivir phosphate and its metabolite Oseltamivir carboxylate using the ex vivo human placenta perfusion model in Chinese Hans population. J Matern Fetal Neonatal Med. 2016 Aug 8:1-5.|[2]de Oliveira JT, et al. Anti-influenza neuraminidase inhibitor Oseltamivir phosphate induces canine mammary cancer cell aggressiveness. PLoS One. 2015 Apr 7;10(4):e0121590.|[3]Li P, et al. A Simple and Robust Approach for Evaluation of Antivirals Using a Recombinant Influenza Virus Expressing Gaussia Luciferase. Viruses. 2018 Jun 13;10(6). pii: E325.
CAS Number
– 204255-11-8
Molecular Weight
– 410.40
Compound Purity
– 99.91
SMILES
– O=C(OCC)C1=C[C@H]([C@@H]([C@H](C1)N)NC(C)=O)OC(CC)CC.OP(O)(O)=O
Clinical Information
– Launched
Research Area
– Infection
Solubility
– DMSO : 100 mg/mL (ultrasonic)|H2O : 100 mg/mL (ultrasonic)
Target
– Influenza Virus
Pathway
– Anti-infection
Product type
– Reference compound
Disclaimer: All products are for Research use only unless clearly stated otherwise on the product datasheet. Datasheets provided on the website are drafts for reference purpose only and you are requested to always refer to the hard copy included in the kit for your experimentation. Agdia Products are available for delivery only in Canada.





