Download Files:
NVP-HSP990
SKU
HY-15190-10 mg
Category Reference compound
Tags Apoptosis;Cell Cycle/DNA Damage;Metabolic Enzyme/Protease, Apoptosis;HSP, Cancer
$83 – $770
Products Details
Product Description
– NVP-HSP990 is a potent, selective and orally active Hsp90 inhibitor, with IC50 values of 0.6, 0.8, and 8.5 nM for Hsp90α, Hsp90β, and Grp94, respectively.
Web ID
– HY-15190
Storage Temperature
– -20°C, 3 years; 4°C, 2 years (Powder)
Shipping
– Room Temperature
Applications
– Cancer-Kinase/protease
Molecular Formula
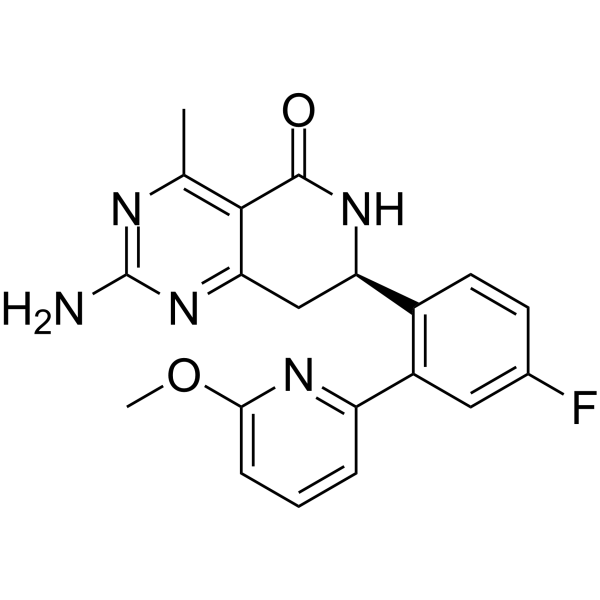
– C20H18FN5O2
References
– [1]Menezes DL, et al. The novel oral Hsp90 inhibitor NVP-HSP990 exhibits potent and broad-spectrum antitumor activities in vitro and in vivo. Mol Cancer Ther. 2012 Mar;11(3):730-9.|[2]McBride CM, et al. Design, structure-activity relationship, and in vivo characterization of the development candidate NVP-HSP990. J Med Chem. 2014 Nov 13;57(21):9124-9.|[3]Lamottke B, et al. The novel, orally bioavailable HSP90 inhibitor NVP-HSP990 induces cell cycle arrest and apoptosis in multiple myeloma cells and acts synergistically with melphalan by increased cleavage of caspases. Eur J Haematol. 2012 May;88(5):406-15.
CAS Number
– 934343-74-5
Molecular Weight
– 379.39
Compound Purity
– 99.77
SMILES
– CC1=C2C(C[C@H](C3=C(C4=NC(OC)=CC=C4)C=C(F)C=C3)NC2=O)=NC(N)=N1
Clinical Information
– Phase 1
Research Area
– Cancer
Solubility
– DMSO : ≥ 33 mg/mL
Target
– Apoptosis;HSP
Isoform
– HSP90
Pathway
– Apoptosis;Cell Cycle/DNA Damage;Metabolic Enzyme/Protease
Product type
– Reference compound
Disclaimer: All products are for research use only unless clearly stated otherwise on the product datasheet. Datasheets provided on the website are drafts for reference purpose only and you are requested to always refer to the hard copy included in the kit for your experimentation.






