Download Files:
Novobiocin
$70
Only 1000 item(s) left in stock.
Products Details
Product Description
– Novobiocin (Albamycin) is a potent and orally active antibiotic. Novobiocin also is a DNA gyrase inhibitor and a heat shock protein 90 (Hsp90) antagonist. Novobiocin has the potential for the research of highly beta-lactam-resistant pneumococcal infections. Novobiocin shows anti-orthopoxvirus activity[1][2][3][4][6].
Web ID
– HY-B0425
Storage Temperature
– -20°C (Powder, sealed storage, away from moisture)
Shipping
– Blue Ice
Applications
– COVID-19-anti-virus
Molecular Formula
– C31H36N2O11
Citations
– Mol Pharm. 2022 Oct 21.|Nat Methods. 2023 Jul 20.|Adv Sci (Weinh). 2022 Oct 18;e2203088.|Blood. 2018 Jul 19;132(3):307-320.|Int J Mol Sci. 2019 Mar 5;20(5). pii: E1125.
References
– [1]Marcu MG, et al. The heat shock protein 90 antagonist novobiocin interacts with a previously unrecognized ATP-binding domain in the carboxyl terminus of the chaperone. J Biol Chem. 2000 Nov 24;275(47):37181-6.|[2]Ali-Osman F, et al. Topoisomerase II inhibition and altered kinetics of formation and repair of nitrosourea and cisplatin-induced DNA interstrand cross-links and cytotoxicity in human glioblastoma cells. Cancer Res. 1993 Dec 1;53(23):5663-8.|[3]Rodríguez-Cerrato V, et al. Comparative efficacy of novobiocin and amoxicillin in experimental sepsis caused by beta-lactam-susceptible and highly resistant pneumococci. Int J Antimicrob Agents. 2010 Jun;35(6):544-9.|[4]Eder JP, et al. A phase I clinical trial of novobiocin, a modulator of alkylating agent cytotoxicity. Cancer Res. 1991 Jan 15;51(2):510-3. |[5]Bhatia S, et al. Targeting HSP90 dimerization via the C terminus is effective in imatinib-resistant CML and lacks the heat shock response.Blood. 2018 Jul 19;132(3):307-320.|[6]Smee DF. Progress in the discovery of compounds inhibiting orthopoxviruses in animal models. Antivir Chem Chemother. 2008;19(3):115-24. |[7]Smee DF. Progress in the discovery of compounds inhibiting orthopoxviruses in animal models. Antivir Chem Chemother. 2008;19(3):115-24.
CAS Number
– 303-81-1
Molecular Weight
– 612.62
Compound Purity
– 94.0
SMILES
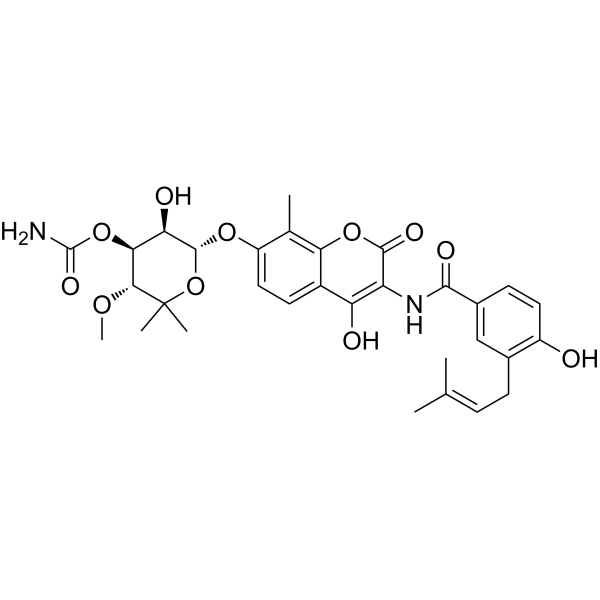
– CC1=C(O2)C(C(O)=C(NC(C3=CC(C/C=C(C)/C)=C(O)C=C3)=O)C2=O)=CC=C1O[C@H]4[C@@H]([C@@H]([C@@H](OC)C(C)(C)O4)OC(N)=O)O
Clinical Information
– Launched
Research Area
– Cancer; Infection
Solubility
– 10 mM in DMSO
Target
– Antibiotic;Apoptosis;Bacterial;DNA/RNA Synthesis;HSP;Orthopoxvirus
Isoform
– HSP90;β-lactam
Pathway
– Anti-infection;Apoptosis;Cell Cycle/DNA Damage;Metabolic Enzyme/Protease
Product type
– Reference compound
Disclaimer: All products are for Research use only unless clearly stated otherwise on the product datasheet. Datasheets provided on the website are drafts for reference purpose only and you are requested to always refer to the hard copy included in the kit for your experimentation. Agdia Products are available for delivery only in Canada.






