Download Files:
nAChR agonist 1
SKU
HY-133011-1 mg
Category Reference compound
Tags Membrane Transporter/Ion Channel;Neuronal Signaling, nAChR, Neurological Disease
$214 – $2,550
Products Details
Product Description
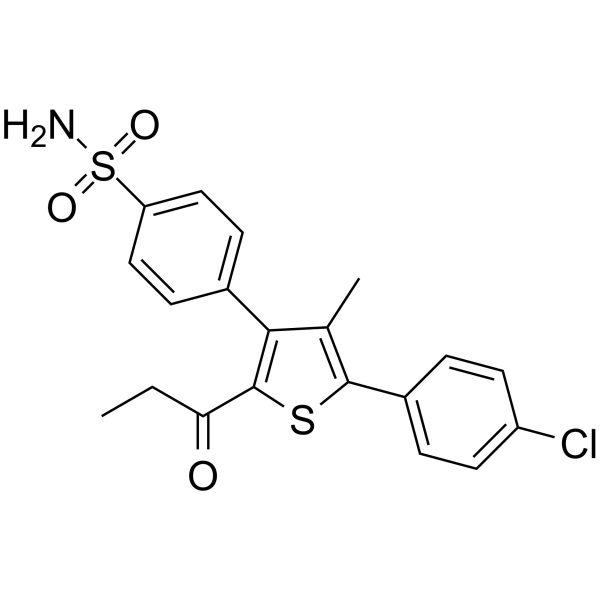
– nAChR agonist 1 is a potent, brain-permeable, and orally efficacious positive allosteric modulator of α7 nicotinic acetylcholine receptor (α7 nAChR). nAChR agonist 1 has the EC50 of 0.32 µM in a Ca2+ mobilization assay (PNU-282987-induced, FLIPR based) in human IMR-32 neuroblastoma cells that endogenously express α7 nAChR. nAChR agonist 1 can be develpoped for the treatment of Alzheimer’s disease[1].
Web ID
– HY-133011
Storage Temperature
– -20°C, 3 years; 4°C, 2 years (Powder)
Shipping
– Room Temperature
Applications
– Neuroscience-Neurodegeneration
Molecular Formula
– C20H18ClNO3S2
References
– [1]Sinha N, et al.Discovery of Novel, Potent, Brain-Permeable, and Orally Efficacious Positive Allosteric Modulator of α7 Nicotinic Acetylcholine Receptor [4-(5-(4-Chlorophenyl)-4-methyl-2-propionylthiophen-3-yl)benzenesulfonamide]: Structure-Activity Relationship and Preclinical Characterization. J Med Chem. 2020 Feb 13;63(3):944-960.
CAS Number
– 1394371-75-5
Molecular Weight
– 419.94
Compound Purity
– 98.35
SMILES
– NS(C1=CC=C(C2=C(C(CC)=O)SC(C3=CC=C(Cl)C=C3)=C2C)C=C1)(=O)=O
Clinical Information
– Phase 1
Research Area
– Neurological Disease
Solubility
– DMSO : 125 mg/mL (ultrasonic)
Target
– nAChR
Pathway
– Membrane Transporter/Ion Channel;Neuronal Signaling
Product type
– Reference compound
Disclaimer: All products are for Research use only unless clearly stated otherwise on the product datasheet. Datasheets provided on the website are drafts for reference purpose only and you are requested to always refer to the hard copy included in the kit for your experimentation. Agdia Products are available for delivery only in Canada.






