Download Files:
Monensin sodium salt
SKU
HY-N0150-100 mg
Category Natural Products
Tags Anti-infection;Membrane Transporter/Ion Channel, Antibiotic;Bacterial;Na+/H+ Exchanger (NHE);Parasite, Infection; Cancer
$50 – $55
Products Details
Product Description
– Monensin sodium salt is an antibiotic secreted by the bacteria Streptomyces cinnamonensis. Monensin sodium salt is an ionophore that mediates Na+/H+ exchange. Monensin sodium salt causes a marked enlargement of the multivesicular bodies (MVBs) and regulates exosome secretion[1][2][3][4].
Web ID
– HY-N0150
Storage Temperature
– 4°C (Powder, sealed storage, away from moisture)
Shipping
– Room Temperature
Applications
– COVID-19-immunoregulation
Molecular Formula
– C36H61NaO11
Citations
– bioRxiv. 2023 Sep 12.|Front Microbiol. 2020 Apr 30;11:790.|Int Immunopharmacol. 2023 Sep 1;124(Pt A):110865.|J Exp Med. 2023 Mar 6;220(3):e20221316.|Nat Commun. 2022 Jul 22;13(1):4255.|Research Square Preprint. 2023 Aug 31.|AAPS PharmSciTech. 2023 Feb 15;24(2):69.|Clin Immunol. 2022: 109217.|Int J Parasitol Drugs Drug Resist. 2023 Aug, 22, 44-51.|J Exp Clin Cancer Res. 2021 Jan 6;40(1):16.|Nat Methods. 2023 Dec 4.|OncoImmunology. 2023 Apr 19.|Redox Biol. 2022 Aug 27;56:102456.|Signal Transduct Target Ther. 2023 Jul 17;8(1):273.|Small. 2021 Nov 1;e2103984.
References
– [1]Ariel Savina, et al. Rab11 promotes docking and fusion of multivesicular bodies in a calcium-dependent manner. Traffic. 2005 Feb;6(2):131-43.|[2]Youhua Huang, et al. Autophagy Participates in Lysosomal Vacuolation-Mediated Cell Death in RGNNV-Infected Cells. Front. Microbiol., 30 April 2020.|[3]Dayekh K, et al. Monensin inhibits epidermal growth factor receptor trafficking and activation: synergistic cytotoxicity in combination with EGFR inhibitors. Mol Cancer Ther. 2014 Nov;13(11):2559-71.|[4]Tumova L, et al. Monensin inhibits canonical Wnt signaling in human colorectal cancer cells and suppresses tumor growth in multiple intestinal neoplasia mice. Mol Cancer Ther. 2014 Apr;13(4):812-22.
CAS Number
– 22373-78-0
Molecular Weight
– 692.85
Compound Purity
– 98.0
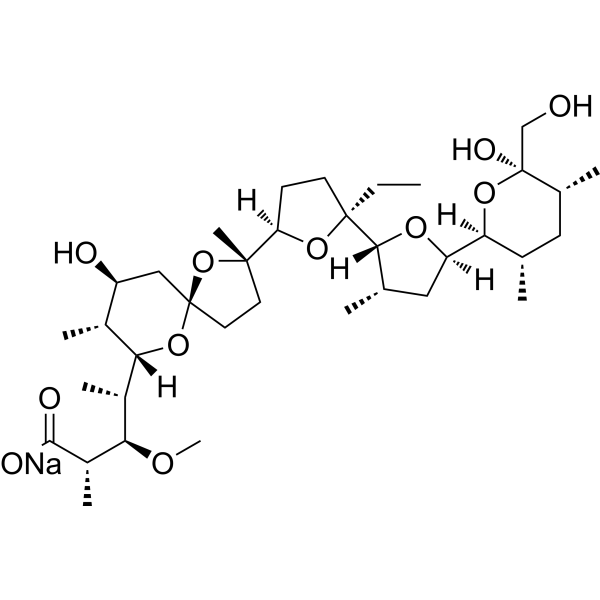
SMILES
– OC[C@@]1(O)[C@H](C)C[C@H](C)[C@]([C@@]2(O[C@]([C@@]3(CC[C@@]([C@]4(O[C@@]5(C[C@H](O)[C@@H](C)[C@@]([H])([C@H]([C@@H](OC)[C@H](C)C(O[Na])=O)C)O5)CC4)C)([H])O3)CC)([H])[C@@H](C)C2)[H])([H])O1
Clinical Information
– No Development Reported
Research Area
– Infection; Cancer
Solubility
– Ethanol : 20 mg/mL (ultrasonic)
Target
– Antibiotic;Bacterial;Na+/H+ Exchanger (NHE);Parasite
Pathway
– Anti-infection;Membrane Transporter/Ion Channel
Product type
– Natural Products
Disclaimer: All products are for Research use only unless clearly stated otherwise on the product datasheet. Datasheets provided on the website are drafts for reference purpose only and you are requested to always refer to the hard copy included in the kit for your experimentation. Agdia Products are available for delivery only in Canada.