Download Files:
MK-2206 (dihydrochloride)
SKU
HY-10358-10 mg
Category Reference compound
Tags Akt;Apoptosis;Autophagy, Apoptosis;Autophagy;PI3K/Akt/mTOR, Cancer
$60 – $450
Products Details
Product Description
– MK-2206 dihydrochloride (MK-2206 (2HCl)) is an orally active allosteric AKT inhibitor with IC50s of 5 nM, 12 nM, and 65 nM for AKT1, AKT2, and AKT3, respectively. MK-2206 dihydrochloride induces autophagy[1][3].
Web ID
– HY-10358
Storage Temperature
– 4°C (Powder, sealed storage, away from moisture)
Shipping
– Room Temperature
Applications
– Cancer-Kinase/protease
Molecular Formula
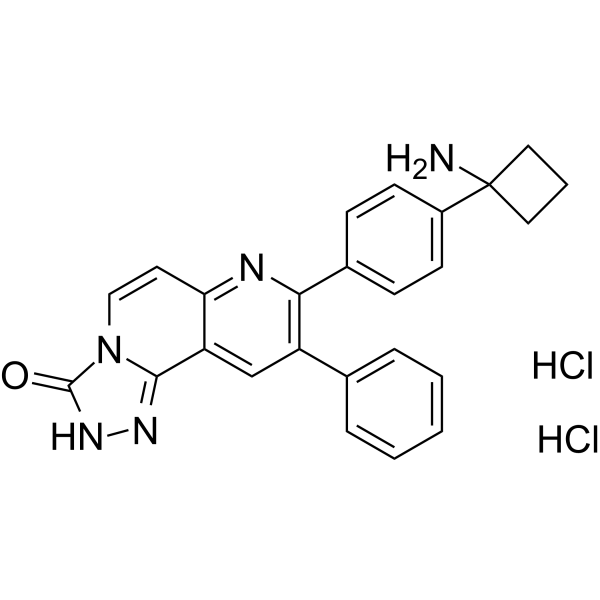
– C25H23Cl2N5O
Citations
– ACS Omega. 2020 Feb 13;5(7):3709-3716.|Acta Otolaryngol. 2021 Dec 28;1-7.|Acta Pharm Sin B. 2021 Jan;11(1):71-88.|Acta Pharm Sin B. 2021 Jun;11(6):1592-1606.|Acta Pharmacol Sin. 2018 Nov;39(11):1787-1796.|Aging. 2020 Jun 12;12(12):11717-11731.|Aging. 2021 Apr 11;13(7):10672-10687.|Am J Cancer Res. 2021 Apr 15;11(4):1557-1571.|Ann Transl Med. 2021 Apr;9(7):533.|Bioact Mater. 2022 Mar 24;18:116-127.|Biochem Biophys Res Commun. 2017 Aug 5;489(4):484-489.|Biochem Biophys Res Commun. 2020 Dec 12;534:121-127.|Biochem Biophys Res Commun. 2022 Jun 25;610:170-175.|Biochem Biophys Res Commun. 3 October 2022.|Biochem Biophys Res Commun. 2019 Feb 26;510(1):97-103. |Biol Trace Elem Res. 2022 Mar 21.|Biomed Pharmacother. 2019 Dec;120:109498.|Biomed Pharmacother. 2021, 111089.|bioRxiv. 2020 Mar.|Biosci Rep. 2019 Dec 20;39(12):BSR20191041.|Bosn J Basic Med Sci. 2021 Aug 1;21(4):434-446.|Br J Cancer. 2017 Sep 26;117(7):974-983.|Brain Dev. 2023 Nov 15:S0387-7604(23)00171-7.|Cancer Biol Ther. 2022 Jan 9;1-14.|Cancer Cell Int. 2021 Apr 26;21(1):235.|Cancer Cell Int. 2022 Jan 11;22(1):18.|Cancer Cell. 2018 Jun 11;33(6):1061-1077.e6. |Cancer Lett. 2020 Apr 10;475:53-64.|Cancer Lett. 2021 Apr 21;S0304-3835(21)00172-5.|Cancer Lett. 2022 Jul 12;215826.|Cancer Res. 2021 Mar 8;canres.3232.2020.|Cancer Sci. 2018 Apr;109(4):944-955. |Cell Death Differ. 2021 Mar;28(3):1026-1040.|Cell Death Differ. 2023 Jan 13.|Cell Death Dis. 2018 Oct 3;9(10):1015. |Cell Death Dis. 2019 May; 10(5): 329.|Cell Death Dis. 2022 Apr 19;13(4):370.|Cell Death Dis. 2022 Sep 29;13(9):838.|Cell Death Dis. 2019 Aug 13;10(8):609. |Cell Immunol. 11 January 2022, 104475.|Cell Mol Gastroenterol Hepatol. 2021;11(3):683-696.|Cell Oncol. 2021 Jul 28.|Cell Prolif. 2021 May 5;e13045.|Cell Rep. 2022 Dec 27;41(13):111891.|Cell Rep. 2023, 42(1): 111916.|Cell Reprogram. 2021 Oct 22.|Cell Signal. 2020 Dec 25;109900.|Cell Stem Cell. 2019 Dec 5;25(6):754-767.e9. |Cell Syst. 2020 Jan 22;10(1):66-81.e11. |Cell. 2014 Feb 13;156(4):771-85.|Cells. 2022 Nov 5;11(21):3505.|Chem Biol. 2012 Jan 27;19(1):140-54.|Clin Epigenetics. 2018 May 23;10:69.|Clin Exp Pharmacol Physiol. 2019 Sep;46(9):861-871.|Curr Med Sci. 2022 Apr;42(2):387-396.|Cytokine. 2020 May;129:155046.|Department of Dental Pharmacology. Okayama University. 2015.|Department of Laboratory Medicine, Laboratory of Hematology of the Radboudumc and Radboud Institute for Molecular Life Sciences in Nijmegen, The Netherlands.2019 Oct.|Dev Growth Differ. 2016 Apr;58(3):280-92.|Elife. 2021 Jul 13;10:e66942.|Endocr Relat Cancer. 2019 Aug;26(8):699-712. |Eur J Cancer. 2022 May 19;170:91-102.|Eur J Pharmacol. 2019 May 15;851:69-79. |Exp Cell Res. 2020 Dec 1;397(1):112341.|Exp Eye Res. 2018 Jul;172:10-20.|Exp Ther Med. 2019 May;17(5):3530-3538. |Exp Ther Med. July 29, 2021.|FASEB J. 2020 Jan;34(1):1481-1496.|FEBS J. 2019 Apr;286(7):1305-1318.|FEBS Open Bio. 2021 May 1.|Food Chem Toxicol. 2018 Oct;120:500-509.|Food Funct. 2021 Mar 9.|Free Radic Biol Med. 2022 Oct 19;S0891-5849(22)00930-3.|Front Cardiovasc Med. 2021 Sep 3;8:687540.|Front Cell Dev Biol. 2021 Apr 14;9:649277.|Front Microbiol. 12 September 2022.|Front Mol Biosci. 08 July 2021.|Genes Dis. 2021 Aug 17;9(2):562-575.|Graduate School of Arts & Sciences. Harvard University. 2016 Aug.|Harvard Medical School LINCS LIBRARY|Hum Mol Genet. 2017 Sep 15;26(18):3553-3563.|In Vitro Cell Dev Biol Anim. 2021 Oct 28.|Int Immunopharmacol. 2021 Jan 30;93:107395.|Int Immunopharmacol. 2021 Oct 26;101(Pt A):108264.|Int J Biochem Cell Biol. 2018 Apr;97:16-27.|Int J Biochem Cell Biol. 2018 Jun;99:43-51. |Int J Biol Sci. 2021 Apr 24;17(7):1821-1836.|Int J Clin Exp Med. 2016;9(9):18513-18518.|Int J Clin Exp Pathol. 2017;10(3):3033-3042.|Int J Mol Sci. 2022 Aug 8;23(15):8798.|Int J Mol Sci. 2023 Jul 6, 24(13), 11168.|Int J Oncol. 2017 Aug;51(2):625-632. |iScience. 2021 Sep 25;24(10):103173.|J Agric Food Chem. 2022 Feb 1.|J Agric Food Chem. 2019 Sep 4;67(35):9805-9811.|J Biochem Mol Toxicol. 2019 Nov;33(11):e22391.|J Biol Chem. 2012 Mar 23;287(13):9742-52.|J Cancer. 2018 Jun 14;9(14):2480-2491. |J Cell Biochem. 2018 May;119(5):3885-3891.|J Cell Biochem. 2019 Oct;120(10):17744-17756.|J Cell Biol. 2021 Oct 4;220(10):e202010118.|J Cell Mol Med. 2020 Jun;24(12):6869-6882.|J Cell Mol Med. 2021 Sep 30.|J Cell Physiol. 2021 Aug;236(8):5818-5831.|J Ethnopharmacol. 2021, 114224.|J Ethnopharmacol. 24 December 2021, 114937.|J Exp Clin Cancer Res. 2021 Aug 20;40(1):262.|J Exp Clin Cancer Res. 2021 Dec 10;40(1):390.|J Exp Clin Cancer Res. 2018 Apr 3;37(1):77. |J Exp Clin Cancer Res. 2019 Feb 13;38(1):76.|J Immunol. 2020 Oct 15;205(8):2255-2264.|J Invest Dermatol. 2019 Jan;139(1):71-80.|J Mater Sci Technol. 13 August 2022.|J Mol Histol. 2019 Aug;50(4):369-374. |J Neuroinflammation. 2023 Feb 24;20(1):49.|J Pain Res. 2020 Dec 1;13:3195-3206.|J Reprod Immunol. 2023 Mar 5;157:103928.|J Transl Med. 2022 Jul 21;20(1):325.|J Transl Med. 2023 Feb 3;21(1):74.|Life Sci Alliance. 2022 Jun 2;5(10):e202101330.|Life Sci. 2020 Mar 15;245:117328.|Life Sci. 2020 May 15;249:117503.|Life Sci. 2020 Nov 1;260:118470.|Life Sci. 2022 Aug 26;120907.|Mediat Inflamm. 2019 Oct 7;2019:6168340.|Modern Oncology. 2017,25(01):0009-0013.|Mol Biol Rep. 2021 Nov 3.|Mol Cancer. 2019 Nov 21;18(1):167. |Mol Cell Biochem. 2023 Sep 22.|Mol Cell. 2021 Jan 21;81(2):370-385.e7.|Mol Cell. 2022 May 13;S1097-2765(22)00393-8.|Mol Med Rep. 2022 Jan;25(1):17.|Mol Oncol. 2022 Jul 19.|Mol Pharmacol. 2019 Dec;96(6):862-870.|Mol Ther Nucleic Acids. 20 July 2022.|Mol Ther Nucleic Acids. May 20, 2022.|Mol Cancer Res. 2020 Mar;18(3):414-423.|Nat Chem Biol. 2017 Jan;13(1):38-45.|Nat Commun. 2018 May 8;9(1):1816. |Nat Commun. 2021 Jun 30;12(1):4050.|Nat Commun. 2022 Aug 2;13(1):4495.|Nature. 2018 Aug;560(7719):499-503.|Neurobiol Dis. 2019 Apr;124:520-530.|Nutrients. 2022, 14(16), 3395.|Oncogene. 2017 Aug 10;36(32):4585-4596.|Oncogene. 2018 Nov;37(45):5997-6009. |Oncogene. 2019 Jun;38(26):5250-5264.|Oncoimmunology. 2018 Aug 6;7(10):e1488565. |Oncol Lett. 2020 Dec;20(6):398.|Oncol Rep. 2019 Aug;42(2):849-856. |Oncotarget. 2016 May 17;7(20):29131-42. |Oncotarget. 2017 Jan 31;8(5):8536-8549. |Oncotarget. 2017 Jul 18;8(29):47642-47654. |Patent. US20140309249A1.|Patent. US20160368910A1.|Patent. US20190248778A1.|Patent. US20220288067A1.|PeerJ. October 25, 2022.|Peptides. 2018 Sep;107:39-44. |Phytomedicine. 2023 Apr 26;115:154833.|Phytomedicine. 23 August 2021, 153687.|Platelets. 2023 Feb 22;2173505.|PLoS One. 2016 Jan 28;11(1):e0147682. |Proc Natl Acad Sci U S A. 2016 Jul 26;113(30):E4338-47. |Proc Natl Acad Sci U S A. 2019 Feb 19;116(8):2996-3005. |Radiat Res. 2018 Aug;190(2):204-215. |Redox Biol. 2021 Dec 18;49:102217.|Reproduction. 2022 Feb 1;REP-21-0282.|Research Square Preprint. 2020 Nov.|Research Square Preprint. 2022 Feb.|Research Square Preprint. 2023 May 5.|Research Square Print. August 22nd, 2022.|Ruperto Carola University Heidelberg. 2023 Jun 1.|Sci Adv. 2022 Jan 21;8(3):eabh2635.|Sci Rep. 2020 May 7;10(1):7714.|Sci Rep. 2022 Aug 13;12(1):13796.|Sci Rep. 2018 Oct 18;8(1):15454|Sci Total Environ. 2021 Nov 10;794:148732.|Sci Transl Med. 2018 Jul 18;10(450):eaaq1093.|Science. 2022 Jul 8;377(6602):eabg9302.|Shock. 2023 Jul 1;60(1):100-109.|Signal Transduct Target Ther. 2021 Jun 18;6(1):234.|Stem Cell Res Ther. 2021 Jan 26;12(1):88.|Stem Cell Res Ther. 2022 Jan 10;13(1):13.|Stem Cells. 2019 Dec;37(12):1567-1580.|Theranostics. 2020 Aug 6;10(21):9899-9912.|Theranostics. 2020 Feb 3;10(6):2859-2871.|Theranostics. 2021 Jan 19;11(7):3392-3416.|Theranostics. 2018 Feb 15;8(7):2044-2060. |Toxicol Appl Pharmacol. 2022 Mar 5;115969.|Toxicology. 2022 Oct;480:153326.|Virology. 2022 Sep 26;576:83-95.|ACS Omega. 2023 Jun 16.|Acta Pharmacol Sin. 2023 May 24.|Am J Physiol Gastrointest Liver Physiol. 2022 May 31.|Antioxidants (Basel). 2022 Oct 18;11(10):2050.|Arch Biochem Biophys. 2023 Apr 1;109595.|Biochem Biophys Res Commun. 2022.|Biochem Biophys Res Commun. 2023 Nov 5, 680, Pages 127-134.|bioRxiv. 2023 May 14.|Blood Adv. 2023 Mar 15;bloodadvances.2022009249.|BMC Complement Med Ther. 2023 Oct 10;23(1):358.|Bone. 2021 Oct 12;116231.|Brain Res Bull. 2020 Sep;162:166-179.|Brain Res Bull. 2023 Mar 15;196:46-58.|Cancer Lett. 2023 Jan 18;556:216063.|Cancers. 2020 Jul 16;12(7):1918.|Carbohydr Polym. 2024 Feb 15, 326, 121637.|Cell Cycle. 2022 Aug 25;1-12.|Cell Death Dis. 2020 May 11;11(5):353. |Cell Death Discov. 2020 Jul 6;6:56.|Cell Mol Immunol. 2023 Jan 5.|Cell Rep. 2022 Feb 15;38(7):110392.|Cell Rep. 2023 Oct 17;42(10):113270.|Cell Rep. 2023 Sep 1;42(9):113048.|Cell Signal. 2023 Oct 20:110933.|Clin Exp Hypertens. 2023 Dec 31;45(1):2208777.|Cytotherapy. 2023 Apr 29;S1465-3249(23)00093-2.|Environ Toxicol. 2023 Aug 16.|Eur J Pharmacol. 2023 Aug 3;956:175957.|Exp Mol Med. 2022 Nov 24.|Exp Ther Med. 2022 Jun;23(6):413.|Exp Ther Med. February 22, 2022.|FASEB J. 2023 Feb;37(2):e22744.|Hematology. 2023 Dec;28(1):2214465.|Indian J Pharm Sci. 2022.|Int Immunopharmacol. 2023 May 7;119:110247.|Int J Biochem Cell Biol. 2023 Feb;155:106359.|Int J Biol Sci. 2023; 19(1): 204-224.|Int J Mol Sci. 2023 Nov 22, 24(23), 16611.|iScience. 2023 Apr 13.|J Biol Chem. 2022 Dec 14;102798.|J Biol Chem. 2022 May 13;102030.|J Biomed Mater Res A. 2020 Aug 28. |J Cancer. 2023 Mar 27;14(5):784-792.|J Ethnopharmacol. 2023 Nov 10:117393.|J Exp Clin Cancer Res. 2022 Jan 26;41(1):38.|J Exp Clin Cancer Res. 2023 Aug 10;42(1):204.|J Nanobiotechnology. 2022 Sep 24;20(1):422.|J Transl Med. 2022 Nov 5;20(1):509.|Life Sci. 2023 Aug 23;122001.|Mol Biol Rep. 2022 Nov 4.|Mol Carcinog. 2023 Apr 12.|Mol Med Rep. 2021 Jul;24(1):508.|Mol Oncol. 2023 Jan 27.|Mol Oncol. 2023 Jul 17.|Molecules. 2022 Dec 5;27(23):8568.|Nat Commun. 2022 Apr 19;13(1):2136.|Nat Commun. 2022 Nov 29;13(1):7345.|Nat Commun. 2023 Sep 28;14(1):6069.|Oncogene. 2020 Oct;39(41):6451-6467.|Oxid Med Cell Longev. 2022|Phytomedicine. 2022 Jul;101:154121.|Phytomedicine. 2023 Jun 23, 154933.|Phytomedicine. 2023 Sep 7, 155074.|R Soc Open Sci. 2020 Jul 8;7(7):200635.|Research Square Preprint. 2021 Aug.|Research Square Preprint. 2023 May 23.|Research Square Preprint. 2023 May 3.|Research Square Preprint. 2023 Oct 11.|Research Square Print. 2023 Mar 9.|Research Square Print. October 11th, 2022.|Sci Signal. 2023 May 16;16(785):eade8111.|Thorac Cancer. 2022 May 10.|Toxicol In Vitro. 2023 Sep 20;105698.|Transl Res. 2023 Nov 4:S1931-5244(23)00181-0.|Transplant Cell Ther. 2023 May 14;S2666-6367(23)01292-7.|University of Zürich. Department of Dermatology. 2021 Dec.
References
– [1]Zhao YY, et al. Effects of an oral allosteric AKT inhibitor (MK-2206) on human nasopharyngeal cancer in vitro and in vivo. Drug Des Devel Ther. 2014 Oct 10;8:1827-37.|[2]Agarwal E, et al. Akt inhibitor MK-2206 promotes anti-tumor activity and cell death by modulation of AIF and Ezrin in colorectal cancer. BMC Cancer. 2014 Mar 1;14:145.|[3]Li Yan, et al. Abstract #DDT01-1: MK-2206: A potent oral allosteric AKT inhibitor. 2009.|[4]Xing Y, et al. Phase II trial of AKT inhibitor MK-2206 in patients with advanced breast cancer who have tumors with PIK3CA or AKT mutations, and/or PTEN loss/PTEN mutation. Breast Cancer Res. 2019 Jul 5;21(1):78.
CAS Number
– 1032350-13-2
Molecular Weight
– 480.39
Compound Purity
– 99.99
SMILES
– O=C1N2C(C3=CC(C4=CC=CC=C4)=C(N=C3C=C2)C5=CC=C(C6(N)CCC6)C=C5)=NN1.Cl.Cl
Clinical Information
– Phase 2
Research Area
– Cancer
Solubility
– DMSO : 12.5 mg/mL (ultrasonic;warming;heat to 60°C)|H2O : 3.57 mg/mL (ultrasonic;warming;heat to 60°C)
Target
– Akt;Apoptosis;Autophagy
Isoform
– Akt1;Akt2;Akt3
Pathway
– Apoptosis;Autophagy;PI3K/Akt/mTOR
Product type
– Reference compound
Disclaimer: All products are for Research use only unless clearly stated otherwise on the product datasheet. Datasheets provided on the website are drafts for reference purpose only and you are requested to always refer to the hard copy included in the kit for your experimentation. Agdia Products are available for delivery only in Canada.






