Download Files:
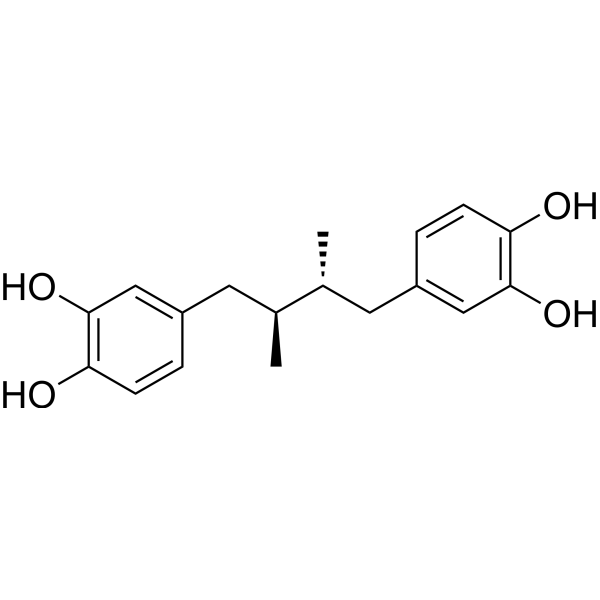
Masoprocol
SKU
HY-109500-10 mg
Category Natural Products
Tags Lipoxygenase, Metabolic Disease, Metabolic Enzyme/Protease
$340 – $540
Products Details
Product Description
– Masoprocol (meso-Nordihydroguaiaretic acid) is a potent and orally active lipoxygenase inhibitor. Masoprocol shows antihyperglycemic activity. Masoprocol decreases the glucose concentration and hepatic triglyceride in vivo. Masoprocol has the potential for the research of type II diabetes[1][2][3].
Web ID
– HY-109500
Storage Temperature
– -20°C (Powder, protect from light)
Shipping
– Blue Ice
Applications
– Metabolism-sugar/lipid metabolism
Molecular Formula
– C18H22O4
References
– [1]Luo J, et al. Masoprocol (nordihydroguaiaretic acid): a new antihyperglycemic agent isolated from the creosote bush (Larrea tridentata). Eur J Pharmacol. 1998 Apr 3;346(1):77-9.|[2]Reed MJ, et al. Effect of masoprocol on carbohydrate and lipid metabolism in a rat model of Type II diabetes. Diabetologia. 1999 Jan;42(1):102-6.|[3]Scribner KA, et al. Masoprocol decreases serum triglyceride concentrations in rats with fructose-induced hypertriglyceridemia. Metabolism. 2000 Sep;49(9):1106-10.
CAS Number
– 27686-84-6
Molecular Weight
– 302.36
Compound Purity
– 99.76
SMILES
– OC(C=C1C[C@@H](C)[C@@H](C)CC2=CC(O)=C(C=C2)O)=C(C=C1)O
Clinical Information
– Launched
Research Area
– Metabolic Disease
Solubility
– DMSO : ≥ 100 mg/mL
Target
– Lipoxygenase
Pathway
– Metabolic Enzyme/Protease
Product type
– Natural Products
Disclaimer: All products are for Research use only unless clearly stated otherwise on the product datasheet. Datasheets provided on the website are drafts for reference purpose only and you are requested to always refer to the hard copy included in the kit for your experimentation. Agdia Products are available for delivery only in Canada.