Download Files:
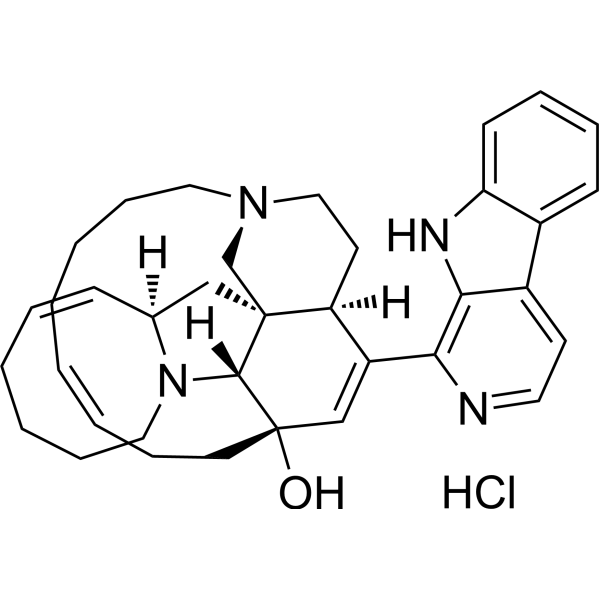
Manzamine A (hydrochloride)
$250 – $800
Products Details
Product Description
– Manzamine A hydrochloride, an orally active beta-carboline alkaloid, inhibits specifically GSK-3β and CDK-5 with IC50s of 10.2 μM and 1.5 μM, respectively. Manzamine A hydrochloride targets vacuolar ATPases and inhibits autophagy in pancreatic cancer cells. Manzamine A hydrochloride has antimalarial and anticancer activities. Manzamine A hydrochloride also shows potent activity against HSV-1[1][2][3][4].
Web ID
– HY-117025A
Storage Temperature
– 4°C (Powder, sealed storage, away from moisture and light)
Shipping
– Room Temperature
Applications
– COVID-19-anti-virus
Molecular Formula
– C36H45ClN4O
References
– [1]Hamann M, et al. Glycogen synthase kinase-3 (GSK-3) inhibitory activity and structure-activity relationship (SAR) studies of the manzamine alkaloids. Potential for Alzheimer’s disease. J Nat Prod. 2007;70(9):1397-1405.|[2]Winkler JD, et al. Antimalarial activity of a new family of analogues of manzamine A. Org Lett. 2006;8(12):2591-2594.|[3]Kallifatidis G, et al. The marine natural product manzamine A targets vacuolar ATPases and inhibits autophagy in pancreatic cancer cells [published correction appears in Mar Drugs. 2014;12(4):2305-7]. Mar Drugs. 2013;11(9):3500-3516. Published 2013 Sep 17.|[4]Palem JR, et al. Manzamine A as a novel inhibitor of herpes simplex virus type-1 replication in cultured corneal cells. Planta Med. 2011;77(1):46-51.|[5]Wahba AE, et al. Structure-activity relationship studies of manzamine A: amidation of positions 6 and 8 of the beta-carboline moiety. Bioorg Med Chem. 2009 Nov 15;17(22):7775-82. |[6]Donia M, et al. Marine natural products and their potential applications as anti-infective agents. Lancet Infect Dis. 2003 Jun;3(6):338-48. |[7]El Sayed KA, et al. New manzamine alkaloids with potent activity against infectious diseases. J Am Chem Soc. 2001 Mar 7;123(9):1804-8.
CAS Number
– 104264-80-4
Molecular Weight
– 585.22
Compound Purity
– 99.29
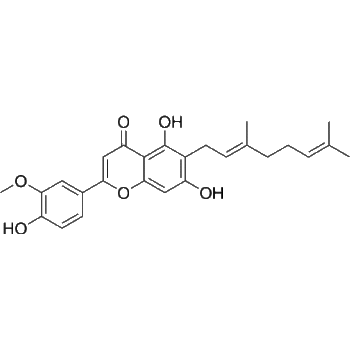
SMILES
– O[C@]1(CC/C=CCCCC2)[C@@]3([H])[C@@](C[C@@]4([H])N3CCCC/C=C4)(C[N@@]2CC5)[C@]5([H])C(C6=C(NC7=CC=CC=C87)C8=CC=N6)=C1.Cl
Clinical Information
– No Development Reported
Research Area
– Cancer; Infection; Neurological Disease
Solubility
– DMSO : 5.88 mg/mL (ultrasonic)
Target
– Autophagy;CDK;GSK-3;HSV;Parasite;Proton Pump
Isoform
– CDK5;GSK-3β;HSV-1;Plasmodium
Pathway
– Anti-infection;Autophagy;Cell Cycle/DNA Damage;Membrane Transporter/Ion Channel;PI3K/Akt/mTOR;Stem Cell/Wnt
Product type
– Natural Products
Disclaimer: All products are for Research use only unless clearly stated otherwise on the product datasheet. Datasheets provided on the website are drafts for reference purpose only and you are requested to always refer to the hard copy included in the kit for your experimentation. Agdia Products are available for delivery only in Canada.