Download Files:
53 CAD – 221 CADPrice range: 53 CAD through 221 CAD
Products Details
Product Description
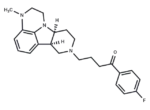
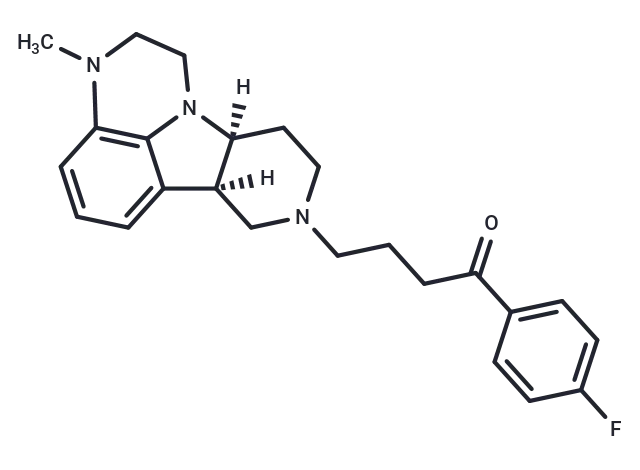
– Lumateperone (ITI 722) is a 5HT2A receptor antagonist and a dopamine receptor phosphoprotein modulator (DPPM).
Web ID
– T21069
Storage Temperature
– -20℃
Shipping
– Blue Ice
Molecular Formula
– C24H28FN3O
References
– Kumar B, Kuhad A, Kuhad A. Lumateperone: a new treatment approach for neuropsychiatric disorders. Drugs Today (Barc). 2018 Dec;54(12):713-719. doi: 10.1358/dot.2018.54.12.2899443. PubMed PMID: 30596390.
CAS Number
– 313368-91-1
Molecular Weight
– C24H28FN3O
Compound Purity
– 0.9977
SMILES
– [H][C@]12CCN(CCCC(=O)c3ccc(F)cc3)C[C@@]1([H])c1cccc3N(C)CCN2c13
Pathway
– Neuroscience|||GPCR/G Protein
Product type
– Small Compound
Disclaimer: All products are for Research use only unless clearly stated otherwise on the product datasheet. Datasheets provided on the website are drafts for reference purpose only and you are requested to always refer to the hard copy included in the kit for your experimentation. Agdia Products are available for delivery only in Canada.
Related Products
1000 in stock
1000 in stock
1000 in stock