Download Files:
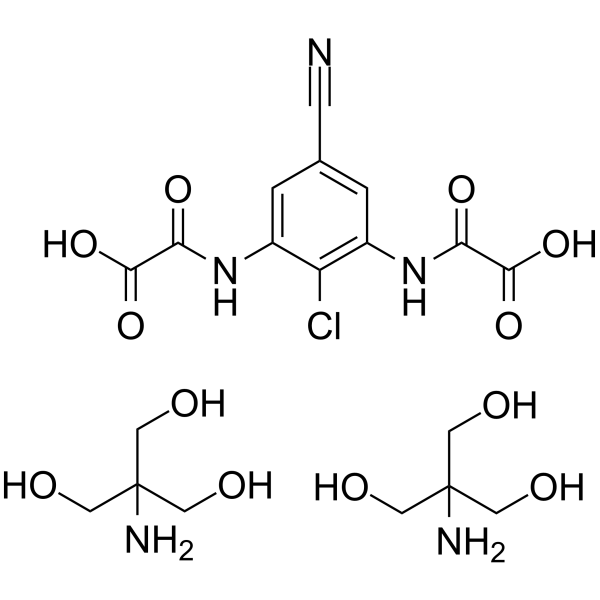
Lodoxamide (tromethamine)
SKU
HY-16289-10 mg
Category Reference compound
Tags GPCR/G Protein;Immunology/Inflammation;Neuronal Signaling, Histamine Receptor, Inflammation/Immunology; Endocrinology
$50 – $240
Products Details
Product Description
– Lodoxamide tromethamine (U-42585E) is a medication for the treatment of prophylaxis of mast cell-mediated allergic disease.
Web ID
– HY-16289
Storage Temperature
– 4°C (Powder, sealed storage, away from moisture)
Shipping
– Room Temperature
Applications
– Neuroscience-Neuromodulation
Molecular Formula
– C19H28ClN5O12
References
– [1]Watt GD, et al. Protective effect opf lodoxamide tromethamine on allergen inhalation challenge. J Allergy Clin Immunol. 1980 Oct;66(4):286-94.|[2]Barr ML, et al. Addition of a mast cell stabilizing compound to organ preservation solutions decreases lung reperfusion injury. J Thorac Cardiovasc Surg. 1998 Mar;115(3):631-6; discussion 636-7.|[3]Mann JS, et al. Inhaled lodoxamide tromethamine in the treatment of perennial asthma: a double-blind placebo-controlled study. J Allergy Clin Immunol. 1985 Jul;76(1):83-90.
CAS Number
– 63610-09-3
Molecular Weight
– 553.90
Compound Purity
– 99.85
SMILES
– N#CC1=CC(NC(C(O)=O)=O)=C(Cl)C(NC(C(O)=O)=O)=C1.OCC(CO)(N)CO.OCC(CO)(N)CO
Clinical Information
– Launched
Research Area
– Inflammation/Immunology; Endocrinology
Solubility
– DMSO : 20 mg/mL (ultrasonic;warming)|H2O : ≥ 100 mg/mL
Target
– Histamine Receptor
Pathway
– GPCR/G Protein;Immunology/Inflammation;Neuronal Signaling
Product type
– Reference compound
Disclaimer: All products are for Research use only unless clearly stated otherwise on the product datasheet. Datasheets provided on the website are drafts for reference purpose only and you are requested to always refer to the hard copy included in the kit for your experimentation. Agdia Products are available for delivery only in Canada.