Download Files:
Lodoxamide
SKU
HY-14270-10 mg
Category Reference compound
Tags GPCR/G Protein;Immunology/Inflammation;Neuronal Signaling, Histamine Receptor, Inflammation/Immunology; Endocrinology
$50 – $420
Products Details
Product Description
– Lodoxamide (U-42585E free acid) is an antiallergic compound acting as a mast-cell stabilizer for the treatment of asthma and allergic conjunctivitis.
Web ID
– HY-14270
Storage Temperature
– -20°C, 3 years; 4°C, 2 years (Powder)
Shipping
– Room Temperature
Applications
– COVID-19-immunoregulation
Molecular Formula
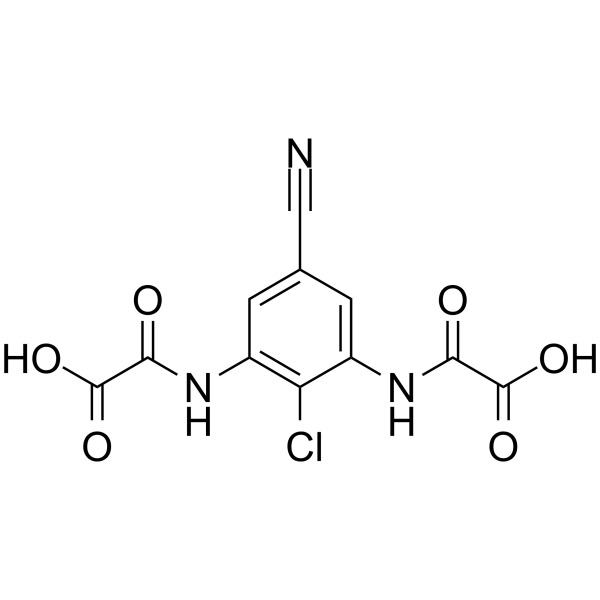
– C11H6ClN3O6
References
– [1]Watt GD, et al. Protective effect opf lodoxamide tromethamine on allergen inhalation challenge. J Allergy Clin Immunol. 1980 Oct;66(4):286-94.|[2]Capron M,et al. Inhibitory effects of lodoxamide on eosinophil activation. Int Arch Allergy Immunol. 1998 Jun;116(2):140-6.|[3]Barr ML, et al. Addition of a mast cell stabilizing compound to organ preservation solutions decreases lung reperfusion injury. J Thorac Cardiovasc Surg. 1998 Mar;115(3):631-6; discussion 636-7.|[4]Mann JS, et al. Inhaled lodoxamide tromethamine in the treatment of perennial asthma: a double-blind placebo-controlled study. J Allergy Clin Immunol. 1985 Jul;76(1):83-90.
CAS Number
– 53882-12-5
Molecular Weight
– 311.63
Compound Purity
– 98.07
SMILES
– OC(C(NC1=C(Cl)C(NC(C(O)=O)=O)=CC(C#N)=C1)=O)=O
Clinical Information
– Launched
Research Area
– Inflammation/Immunology; Endocrinology
Solubility
– DMSO : 50 mg/mL (ultrasonic)
Target
– Histamine Receptor
Pathway
– GPCR/G Protein;Immunology/Inflammation;Neuronal Signaling
Product type
– Reference compound
Disclaimer: All products are for Research use only unless clearly stated otherwise on the product datasheet. Datasheets provided on the website are drafts for reference purpose only and you are requested to always refer to the hard copy included in the kit for your experimentation. Agdia Products are available for delivery only in Canada.






