Download Files:
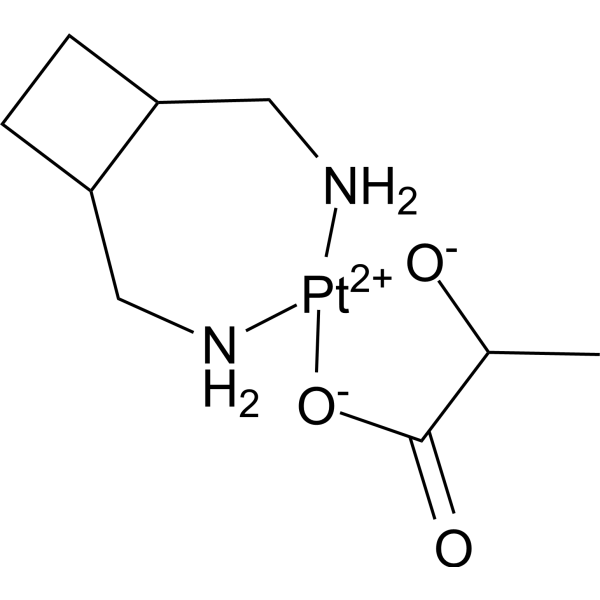
Lobaplatin
SKU
HY-105930-1 mg
Category Reference compound
Tags Apoptosis, Apoptosis;Bcl-2 Family;Caspase, Cancer
$110 – $1,600
Products Details
Product Description
– Lobaplatin (D-19466) is a diastereometric mixture of platinum(II) complexe. Lobaplatin arrests cell cycle at G1 and G2/M phase. Lobaplatin induces apoptosis by increasing expressions of caspase and Bax, decreasing expression of Bcl-2. Lobaplatin can be used for research of cancer[1][2][3].
Web ID
– HY-105930
Storage Temperature
– -20°C, 3 years; 4°C, 2 years (Powder)
Shipping
– Room Temperature
Applications
– Cancer-programmed cell death
Molecular Formula
– C9H18N2O3Pt
References
– [1]Du L, et, al. Antitumor activity of Lobaplatin against esophageal squamous cell carcinoma through caspase-dependent apoptosis and increasing the Bax/Bcl-2 ratio. Biomed Pharmacother. 2017 Nov;95:447-452.|[2]Wu Q, et, al. Lobaplatin arrests cell cycle progression in human hepatocellular carcinoma cells. J Hematol Oncol. 2010 Oct 31;3:43.|[3]McKeage MJ. Lobaplatin: a new antitumour platinum drug. Expert Opin Investig Drugs. 2001 Jan;10(1):119-28.
CAS Number
– 135558-11-1
Molecular Weight
– 397.33
Compound Purity
– 98.0
SMILES
– CC1[O-][Pt+2]2([O-]C1=O)[NH2]CC(CC3)C3C[NH2]2
Clinical Information
– Phase 4
Research Area
– Cancer
Solubility
– DMSO : 4.55 mg/mL (ultrasonic;warming;heat to 60°C)|H2O : 50 mg/mL (ultrasonic;warming;heat to 60°C)
Target
– Apoptosis;Bcl-2 Family;Caspase
Pathway
– Apoptosis
Product type
– Reference compound
Disclaimer: All products are for Research use only unless clearly stated otherwise on the product datasheet. Datasheets provided on the website are drafts for reference purpose only and you are requested to always refer to the hard copy included in the kit for your experimentation. Agdia Products are available for delivery only in Canada.






