Download Files:
Linezolid
$35 – $198
Products Details
Product Description
– Linezolid (PNU-100766) is the first member of the class of oxazolidinone synthetic antibiotic. Linezolid acts by inhibiting the initiation of bacterial protein synthesis. Linezolid is used for the treatment of serious infections caused by Gram-positive bacteria that are resistant to several other antibiotics[1][2][3].
Web ID
– HY-10394
Storage Temperature
– 4°C (Powder, protect from light)
Shipping
– Room Temperature
Applications
– COVID-19-immunoregulation
Molecular Formula
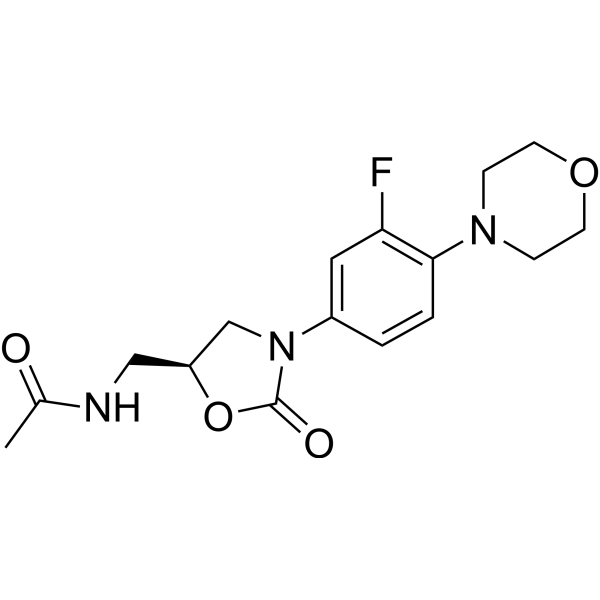
– C16H20FN3O4
References
– [1]Clemett D, Markham A. Linezolid. Drugs. 2000 Apr;59(4):815-27; discussion 828.|[2]Chiappini E, Conti C, Galli L et al. Clinical efficacy and tolerability of linezolid in pediatric patients: a systematic review. Clin Ther. 2010 Jan;32(1):66-88.|[3]Perry CM, Jarvis B. Linezolid: a review of its use in the management of serious gram-positive infections. Drugs. 2001;61(4):525-51.|[4]He MZ, Jiang YW, Cai C.
Mechanisms and epidemiology of linezolid resistance in staphylococci.Zhonghua Jie He He Hu Xi Za Zhi. 2012 May;35(5):360-2.|[5]Karau MJ, Tilahun AY, Schmidt SM, Clark CR, Patel R, Rajagopalan G.
Linezolid is Superior to Vancomycin in Experimental Pneumonia Caused by Superantigen-Producing Staphylococcus aureus in HLA class II Transgenic Mice.
Antimicrob Agents Chemother. 2012 Jul 30.
CAS Number
– 165800-03-3
Molecular Weight
– 337.35
Compound Purity
– 99.95
SMILES
– O=C(O[C@H]1CNC(C)=O)N(C1)C2=CC(F)=C(N3CCOCC3)C=C2
Clinical Information
– Launched
Research Area
– Infection
Solubility
– DMSO : ≥ 100 mg/mL
Target
– Antibiotic;Bacterial
Isoform
– Oxazolidinone
Pathway
– Anti-infection
Product type
– Reference compound
Disclaimer: All products are for Research use only unless clearly stated otherwise on the product datasheet. Datasheets provided on the website are drafts for reference purpose only and you are requested to always refer to the hard copy included in the kit for your experimentation. Agdia Products are available for delivery only in Canada.






