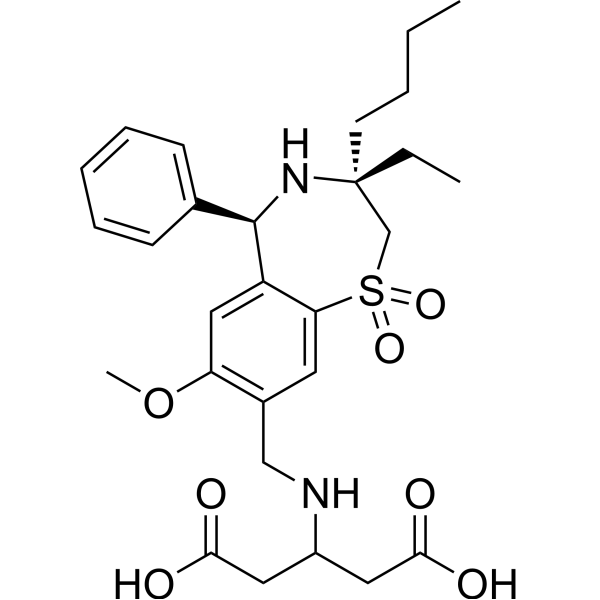
Download Files:
Linerixibat
SKU
HY-16643-1 mg
Category Reference compound
Tags Apical Sodium-Dependent Bile Acid Transporter, Membrane Transporter/Ion Channel, Metabolic Disease; Inflammation/Immunology
$100 – $2,160
Products Details
Product Description
– Linerixibat (GSK2330672) is a highly potent, nonabsorbable and orally active apical sodium-dependent bile acid transporter (ASBT) inhibitor with an IC50 of 42 nM human ASBT. Linerixibat can be used as lipid-lowering agent. Linerixibat has the potential for type 2 diabetes and Primary Biliary Cholangitis treatment[1][2][3].
Web ID
– HY-16643
Storage Temperature
– -20°C, 3 years; 4°C, 2 years (Powder)
Shipping
– Room Temperature
Applications
– Metabolism-sugar/lipid metabolism
Molecular Formula
– C28H38N2O7S
References
– [1]Wang Y, et al. HNF4α Regulates CSAD to Couple Hepatic Taurine Production to Bile Acid Synthesis in Mice. Gene Expr. 2018 Aug 22;18(3):187-196.|[2]Wu Y, et al. Discovery of a highly potent, nonabsorbable apical sodium-dependent bile acid transporter inhibitor (GSK2330672) for treatment of type 2 diabetes. J Med Chem. 2013 Jun 27;56(12):5094-114.|[3]Linerixibat (GSK2330672) granted Orphan Status. September 24, 2019.
CAS Number
– 1345982-69-5
Molecular Weight
– 546.68
Compound Purity
– 99.98
SMILES
– O=C(O)CC(NCC1=C(OC)C=C(C2=C1)[C@@H](C3=CC=CC=C3)N[C@](CC)(CCCC)CS2(=O)=O)CC(O)=O
Clinical Information
– Phase 3
Research Area
– Metabolic Disease; Inflammation/Immunology
Solubility
– DMSO : 50 mg/mL (ultrasonic)
Target
– Apical Sodium-Dependent Bile Acid Transporter
Pathway
– Membrane Transporter/Ion Channel
Product type
– Reference compound
Disclaimer: All products are for Research use only unless clearly stated otherwise on the product datasheet. Datasheets provided on the website are drafts for reference purpose only and you are requested to always refer to the hard copy included in the kit for your experimentation. Agdia Products are available for delivery only in Canada.






