Download Files:
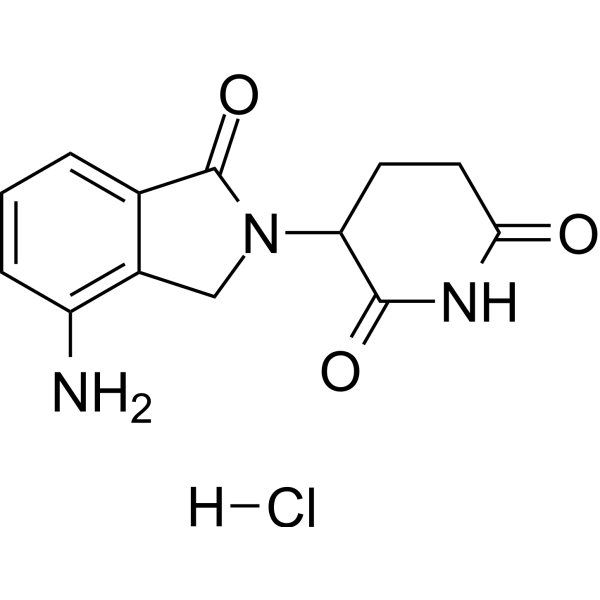
Lenalidomide (hydrochloride)
SKU
HY-A0003A-Get quote
Category Reference compound
Tags Cancer; Inflammation/Immunology, Ligands for E3 Ligase;Molecular Glues, PROTAC
Products Details
Product Description
– Lenalidomide hydrochloride (CC-5013 hydrochloride), a derivative of Thalidomide, acts as molecular glue. Lenalidomide hydrochloride is an orally active immunomodulator. Lenalidomide hydrochloride (CC-5013 hydrochloride) is a ligand of ubiquitin E3 ligase cereblon (CRBN), and it causes selective ubiquitination and degradation of two lymphoid transcription factors, IKZF1 and IKZF3, by the CRBN-CRL4 ubiquitin ligase. Lenalidomide hydrochloride (CC-5013 hydrochloride) specifically inhibits growth of mature B-cell lymphomas, including multiple myeloma, and induces IL-2 release from T cells[1][2].
Web ID
– HY-A0003A
Shipping
– Room temperature
Applications
– COVID-19-immunoregulation
Molecular Formula
– C13H14ClN3O3
Citations
– Food Chem Toxicol. 2019 Mar;125:392-402.|ACS Pharmacol Transl Sci. 2021 Feb 26.|Acta Pharmacol Sin. 2020 Sep;41(9):1246-1254.|Am J Pathol. 2015 Jun;185(6):1783-94. |Biochim Biophys Acta Mol Basis Dis. 2022 Jun 22;166472.|bioRxiv. April.|Cancer Cell. 2022 Aug 26;S1535-6108(22)00372-5.|Cancer Sci. 2019 Dec;110(12):3802-3810.|Cell Syst. 2018 Apr 25;6(4):424-443.e7.|Cell. 2018 Sep 20;175(1):171-185.e25.|Elife. 2018 Aug 1;7:e38430. |Exp Cell Res. 2020 Aug 1;393(1):112054.|Faculty of Science, Masaryk University. Department of Experimental Biology.2014. |Food Chem Toxicol. 2019 Mar;125:392-402.|Free Radic Biol Med. 2023 Apr 10;S0891-5849(23)00373-8.|Front Cell Infect Microbiol. 2022 Jul 28;12:954814.|Harvard Medical School LINCS LIBRARY|Inflammation. 2019 Feb;42(1):221-234. |Int J Mol Sci. 2023 Sep 14, 24(18), 14097.|iScience. 19 July 2022, 104781.|Nat Cancer. 2022 May;3(5):595-613.|Nat Chem Biol. 2021 Jun;17(6):711-717.|Nat Commun. 2017 May 22;8:15398. |Oncol Rep. 2018 Jun;39(6):2873-2880. |Oxid Med Cell Longev. 29 Dec 2021.|Patent. US20170360780A1.|Patent. US20180263995A1.|Patent. US20210379039A1.|Patent. US20220332776A1.|University of Washington. The Columbian College of Arts and Sciences. August 31, 2021.
References
– [1]Omran A, et al. Effects of MRP8, LPS, and lenalidomide on the expressions of TNF-α , brain-enriched, and inflammation-related microRNAs in the primary astrocyte culture. ScientificWorldJournal. 2013 Sep 21;2013:208309.|[2]Rozewski DM, et al. Pharmacokinetics and tissue disposition of lenalidomide in mice. AAPS J. 2012 Dec;14(4):872-82.|[3]Kotla V, et al. Mechanism of action of lenalidomide in hematological malignancies. J Hematol Oncol. 2009 Aug 12;2:36.|[4]Lopez-Girona A, et al. Cereblon is a direct protein target for immunomodulatory and antiproliferative activities of lenalidomide and pomalidomide. Leukemia. 2012 Nov;26(11):2326-35.|[5]Minzel W, et al. Small Molecules Co-targeting CKIα and the Transcriptional Kinases CDK7/9 Control AML in Preclinical Models. Cell. 2018 Sep 20;175(1):171-185.e25.|[6]Krönke J, et al. Lenalidomide induces degradation of IKZF1 and IKZF3. Oncoimmunology. 2014 Jul 3;3(7):e941742.
CAS Number
– 1243329-97-6
Molecular Weight
– 295.72
SMILES
– O=C1C2=CC=CC(N)=C2CN1C(C(N3)=O)CCC3=O.[H]Cl
Clinical Information
– Launched
Research Area
– Cancer; Inflammation/Immunology
Solubility
– 10 mM in DMSO
Target
– Ligands for E3 Ligase;Molecular Glues
Isoform
– Cereblon
Pathway
– PROTAC
Product type
– Reference compound
Disclaimer: All products are for Research use only unless clearly stated otherwise on the product datasheet. Datasheets provided on the website are drafts for reference purpose only and you are requested to always refer to the hard copy included in the kit for your experimentation. Agdia Products are available for delivery only in Canada.






