Download Files:
Lapatinib
SKU
HY-50898-1 g
Category Reference compound
Tags Apoptosis;Autophagy;JAK/STAT Signaling;Protein Tyrosine Kinase/RTK, Autophagy;EGFR;Ferroptosis, Cancer
$66 – $198
Products Details
Product Description
– Lapatinib (GW572016) is a potent inhibitor of the ErbB-2 and EGFR tyrosine kinase domains with IC50 values against purified EGFR and ErbB-2 of 10.2 and 9.8 nM, respectively[1].
Web ID
– HY-50898
Storage Temperature
– -20°C, 3 years; 4°C, 2 years (Powder)
Shipping
– Room Temperature
Applications
– Cancer-Kinase/protease
Molecular Formula
– C29H26ClFN4O4S
Citations
– ACS Chem Biol. 2016 Apr 15;11(4):992-1000.|ACS Omega. March 3, 2022.|Acta Pharmacol Sin. 2021 Jan;42(1):108-114.|Adv Sci (Weinh). 2022 Dec 23;e2203884.|Analyst. 2017 Jan 26;142(3):525-536. |Biol Proced Online. 2023 Jun 27;25(1):19.|Biomedical Engineering Advances. June 2022, 100040.|bioRxiv. 2019 Sep.|bioRxiv. 2021 Jan 9.|Biotechnol Bioeng. 2021 Sep 3.|Br J Cancer. 2021 Nov 24.|Cancer Chemother Pharmacol. 2018 Sep;82(3):383-394.|Cancer Lett. 2020 Apr 10;475:53-64.|Cancer Lett. 2021 Apr 28;504:125-136.|Cancer Lett. 2021 Jun 18.|Cancers (Basel). 2022, 14(23), 5854|Carcinogenesis. 2022 Sep 30;bgac078.|Cell Mol Life Sci. 2023 Mar 18;80(4):100.|Cell Physiol Biochem. 2015;37(6):2275-87.|Cell Syst. 2020 Nov 18;11(5):478-494.e9.|Clin Chim Acta. 2018 Oct;485:298-304.|Clin Transl Med. 2020 Aug;10(4):e148.|Drug Des Devel Ther. 2020 Feb 25;14:783-793.|Eur J Drug Metab Pharmacokinet. 2021 Jul 18;1-11.|Eur J Med Chem. 2019 Aug 15;176:393-409.|Exp Cell Res. 2020 Aug 1;393(1):112054.|Front Immunol. 2023 Mar 17.|Front Oncol. 2021 Mar 26;11:608201.|Front Pharmacol. 2021 Mar 8;12:644342.|Fundam Clin Pharmacol. 2021 Feb 1.|Gene. 2022 Jan 10;146171.|Head Neck. 2023 May 15.|iScience. 2023 Oct 3.|J Biol Chem. 2022 Jul 31;102310.|J Chromatogr B Analyt Technol Biomed Life Sci. 2017 Feb 27;1049-1050:30-40.|J Exp Clin Cancer Res. 2018 Jun 25;37(1):123.|J Nanopart Res. 2022; 24:261|J Proteome Res. 2022 Feb 16.|Life Sci. 2021 Feb 27;119239.|Mol Cancer Ther. 2018 Mar;17(3):603-613.|Mol Pharm. 2022 Oct 21.|Nat Commun. 2023 Apr 13;14(1):2110.|Nat Commun. 2023 Jun 15;14(1):3560.|Nat Immunol. 2018 Mar;19(3):233-245.|Nat Med. 2016 Jul;22(7):723-6.|Nature. 2017 Aug 24;548(7668):471-475. |Oncogene. 2016 Jun 9;35(23):2961-70. |Oncogene. 2022 May;41(22):3064-3078.|Oncotarget. 2017 Aug 24;8(62):104894-104912.|Oncotarget. 2020 Nov 3;11(44):3921-3932.|Open Life Sci. 2023, 18(1).|Patent. US20200108066A1|Patent. US20220305013A1.|Personalized Medicine Universe. 2019 May. |PLoS One. 2019 Apr 4;14(4):e0214598.|RSC Adv. 2019, 9(34):19325-19332.|Sci Transl Med. 2018 Jul 18;10(450):eaaq1093.|Separations. 2023 May 26, 10(6), 330.|Stem Cell Reports. 2017 Dec 12;9(6):1948-1960.|Transl Oncol. 2022 Jul; 21: 101444.|Tumour Biol. 2016 Nov;37(11):14831-14839.|Harvard Medical School LINCS LIBRARY
References
– [1]Rusnak DW, et al. The effects of the novel, reversible epidermal growth factor receptor/ErbB-2 tyrosine kinase inhibitor, GW2016, on the growth of human normal and tumor-derived cell lines in vitro and in vivo. Mol Cancer Ther. 2001 Dec;1(2):85-94
CAS Number
– 231277-92-2
Molecular Weight
– 581.06
Compound Purity
– 99.83
SMILES
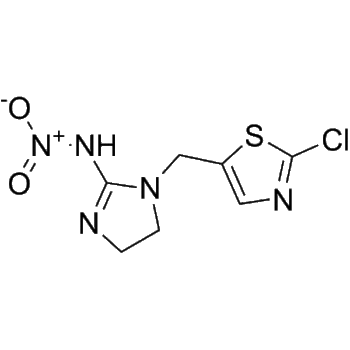
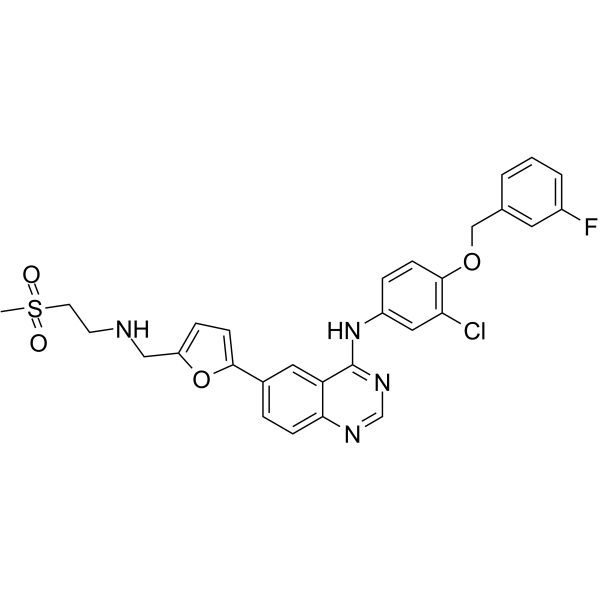
– O=S(CCNCC1=CC=C(C2=CC=C3C(C(NC4=CC(Cl)=C(C=C4)OCC5=CC(F)=CC=C5)=NC=N3)=C2)O1)(C)=O
Clinical Information
– Launched
Research Area
– Cancer
Solubility
– DMSO : 125 mg/mL (ultrasonic)
Target
– Autophagy;EGFR;Ferroptosis
Isoform
– EGFR/ErbB1/HER1;ErbB2/HER2
Pathway
– Apoptosis;Autophagy;JAK/STAT Signaling;Protein Tyrosine Kinase/RTK
Product type
– Reference compound
Disclaimer: All products are for Research use only unless clearly stated otherwise on the product datasheet. Datasheets provided on the website are drafts for reference purpose only and you are requested to always refer to the hard copy included in the kit for your experimentation. Agdia Products are available for delivery only in Canada.