Download Files:
L-368,899 hydrochloride
SKU
HY-108677-1 mg
Category Reference compound
Tags Endocrinology, GPCR/G Protein, Oxytocin Receptor
$110 – $1,500
Products Details
Product Description
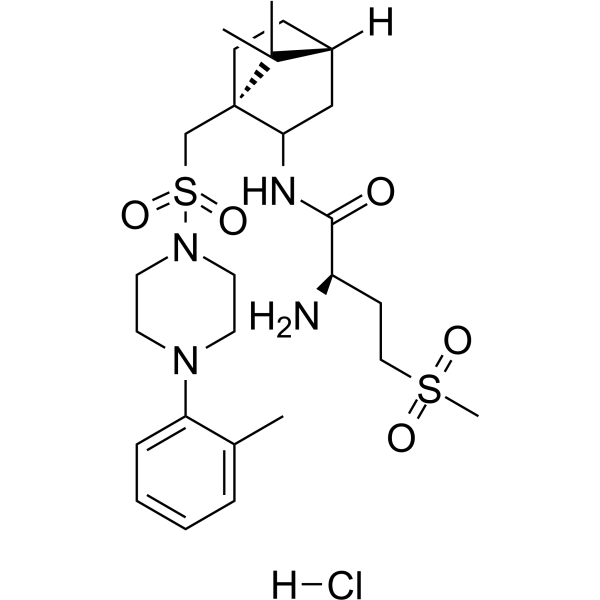
– L-368,899 hydrochloride is a potent, selective, orally bioavailable, non-peptide oxytocin receptor antagonist, with IC50s of 8.9 nM and 26 nM for rat uterus and human uterus oxytocin receptor, respectively. L-368,899 hydrochloride used as a tocolytic agent[1].
Web ID
– HY-108677
Storage Temperature
– 4°C (Powder, sealed storage, away from moisture)
Shipping
– Room Temperature
Molecular Formula
– C26H43ClN4O5S2
References
– [1]Kathryn L. Thompson, et al. Pharmacokinetics and Disposition of the Oxytocin Receptor Antagonist L-368,899 in Rats and Dogs|[2]Williams PD, et al. 1-((7,7-Dimethyl-2(S)-(2(S)-amino-4-(methylsulfonyl)butyramido)bicyclo [2.2.1]-heptan-1(S)-yl)methyl)sulfonyl)-4-(2-methylphenyl)piperaz ine (L-368,899): an orally bioavailable, non-peptide oxytocin antagonist with potential utility for managing preterm labor. J Med Chem. 1994 Mar 4;37(5):565-71.
CAS Number
– 160312-62-9
Molecular Weight
– 591.23
Compound Purity
– 98.61
SMILES
– O=C(NC1[C@@](C2(C)C)(CS(=O)(N3CCN(C4=CC=CC=C4C)CC3)=O)CC[C@]2([H])C1)[C@H](N)CCS(=O)(C)=O.[H]Cl
Clinical Information
– No Development Reported
Research Area
– Endocrinology
Solubility
– DMSO : 130 mg/mL (ultrasonic)|H2O : 50 mg/mL (ultrasonic)
Target
– Oxytocin Receptor
Pathway
– GPCR/G Protein
Product type
– Reference compound
Disclaimer: All products are for Research use only unless clearly stated otherwise on the product datasheet. Datasheets provided on the website are drafts for reference purpose only and you are requested to always refer to the hard copy included in the kit for your experimentation. Agdia Products are available for delivery only in Canada.






