Download Files:
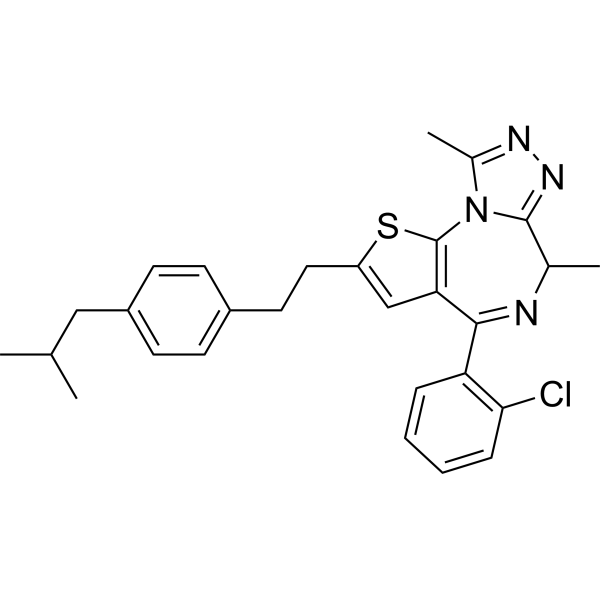
Israpafant
SKU
HY-106837-Get quote
Category Reference compound
Tags GPCR/G Protein, Inflammation/Immunology, Platelet-activating Factor Receptor (PAFR)
Products Details
Product Description
– Israpafant (Y-24180) is a potent, selective and long-acting platelet activation factor (PAF) receptor antagonist with IC50s of 0.84 nM and 3.84 nM against PAF-induced human and rabbit platelet aggregation, respectively. Israpafant stimulates both extracellular Ca2+ influx and intracellular Ca2+ release in prostate cancer cells. Israpafant suppresses the allergic cutaneous reactions including eosinophilia, cytokine production, edema and erythema in mice[1][2].
Web ID
– HY-106837
Shipping
– Room temperature
Molecular Formula
– C28H29ClN4S
References
– [1]Takehara S, et al. Pharmacological actions of Y-24180, a new specific antagonist of platelet activating factor (PAF): II. Interactions with PAF and benzodiazepine receptors. Prostaglandins. 1990 Dec;40(6):571-83.|[2]Jan C R, Chao Y Y. Novel effect of Y-24180, a presumed specific platelet activation factor receptor antagonist, on Ca2+ levels and growth of human prostate cancer cells. Cellular signalling, 2004, 16(8): 959-965.
CAS Number
– 117279-73-9
Molecular Weight
– 489.07
SMILES
– CC1=NN=C2N1C3=C(C(C4=C(Cl)C=CC=C4)=NC2C)C=C(S3)CCC5=CC=C(C=C5)CC(C)C
Clinical Information
– No Development Reported
Research Area
– Inflammation/Immunology
Solubility
– 10 mM in DMSO
Target
– Platelet-activating Factor Receptor (PAFR)
Pathway
– GPCR/G Protein
Product type
– Reference compound
Disclaimer: All products are for Research use only unless clearly stated otherwise on the product datasheet. Datasheets provided on the website are drafts for reference purpose only and you are requested to always refer to the hard copy included in the kit for your experimentation. Agdia Products are available for delivery only in Canada.








