Download Files:
Ioxilan
$200 – $300
Products Details
Product Description
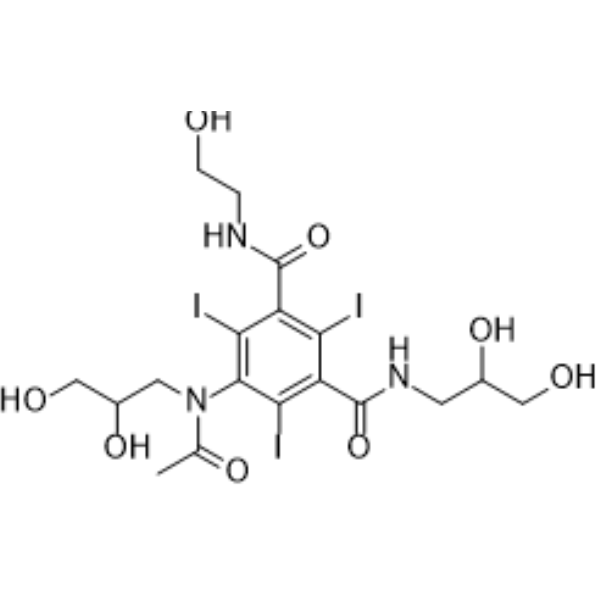
– Ioxilan is a low-osmolar, nonionic and tri-iodinated diagnostic contrast agent. Ioxilan is also an X-ray contrast agent for excretory urography and contrast enhanced computed tomographic (CECT) imaging of the head and body. Intravascular injection results in opacification of vessels in the path of flow of the contrast medium, permitting radiographic visualization of the internal structures of the human body until significant hemodilution occurs[1][2][3].
Web ID
– HY-109513
Storage Temperature
– -20°C, 3 years; 4°C, 2 years (Powder)
Shipping
– Room Temperature
Molecular Formula
– C18H24I3N3O8
References
– [1]Cheng KT. Ioxilan carbonate particles. National Center for Biotechnology Information (US); 2004-2013. 2007 Sep 1.|[2]Chow SL, et al. Effect of iodixanol and ioxilan on QT interval and renal function in patients with systolic heart failure. Int J Cardiol. 2012 Jan 12;154(1):17-21.|[3] Nonionic Contrast Agents.
CAS Number
– 107793-72-6
Molecular Weight
– 791.11
Compound Purity
– 98.34
SMILES
– O=C(C1=C(I)C(N(C(C)=O)CC(O)CO)=C(I)C(C(NCCO)=O)=C1I)NCC(O)CO
Clinical Information
– Launched
Research Area
– Others
Solubility
– DMSO : ≥ 250 mg/mL
Target
– Biochemical Assay Reagents
Pathway
– Others
Product type
– Reference compound
Disclaimer: All products are for Research use only unless clearly stated otherwise on the product datasheet. Datasheets provided on the website are drafts for reference purpose only and you are requested to always refer to the hard copy included in the kit for your experimentation. Agdia Products are available for delivery only in Canada.






