Download Files:
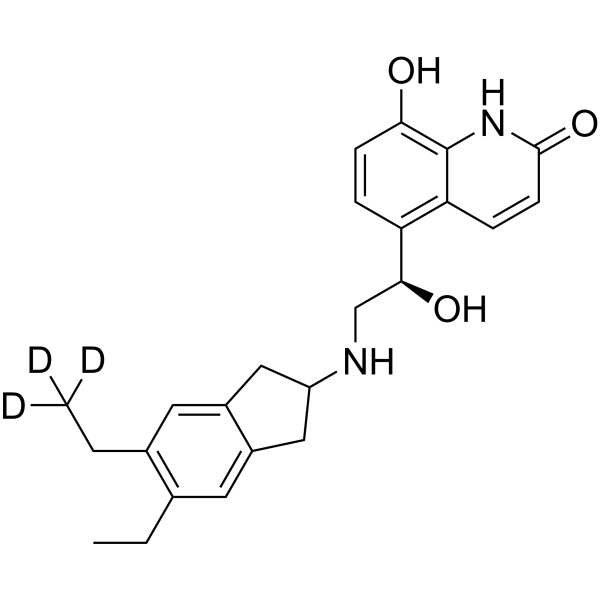
Indacaterol-d3
SKU
HY-14299S-1mg
Category Isotope-Labeled Compounds
Tags Adrenergic Receptor;Isotope-Labeled Compounds, GPCR/G Protein;Neuronal Signaling;Others, Neurological Disease; Endocrinology
Products Details
Product Description
– Indacaterol-d3 is deuterium labeled Indacaterol.
Web ID
– HY-14299S
Shipping
– Room temperature
Applications
– Metabolism-protein/nucleotide metabolism
Molecular Formula
– C24H25D3N2O3
References
– [1]Battram, C., et al., In vitro and in vivo pharmacological characterization of 5-[(R)-2-(5,6-diethyl-indan-2-ylamino)-1-hydroxy-ethyl]-8-hydroxy-1H-quinolin-2-o ne (indacaterol), a novel inhaled beta(2) adrenoceptor agonist with a 24-h duration of action. J Pharmacol Exp Ther, 2006. 317(2): p. 762-70.|[2]Sturton, R.G., et al., Pharmacological characterization of indacaterol, a novel once daily inhaled 2 adrenoceptor agonist, on small airways in human and rat precision-cut lung slices. J Pharmacol Exp Ther, 2008. 324(1): p. 270-5.|[3]Russak EM, et al. Impact of Deuterium Substitution on the Pharmacokinetics of Pharmaceuticals. Ann Pharmacother. 2019;53(2):211-216.
CAS Number
– 2699828-16-3
Molecular Weight
– 395.51
SMILES
– CCC(C=C(C1)C(CC1NC[C@@H](C2=C3C(NC(C=C3)=O)=C(C=C2)O)O)=C4)=C4CC([2H])([2H])[2H]
Clinical Information
– No Development Reported
Research Area
– Neurological Disease; Endocrinology
Solubility
– 10 mM in DMSO
Target
– Adrenergic Receptor;Isotope-Labeled Compounds
Pathway
– GPCR/G Protein;Neuronal Signaling;Others
Product type
– Isotope-Labeled Compounds
Disclaimer: All products are for Research use only unless clearly stated otherwise on the product datasheet. Datasheets provided on the website are drafts for reference purpose only and you are requested to always refer to the hard copy included in the kit for your experimentation. Agdia Products are available for delivery only in Canada.