Download Files:
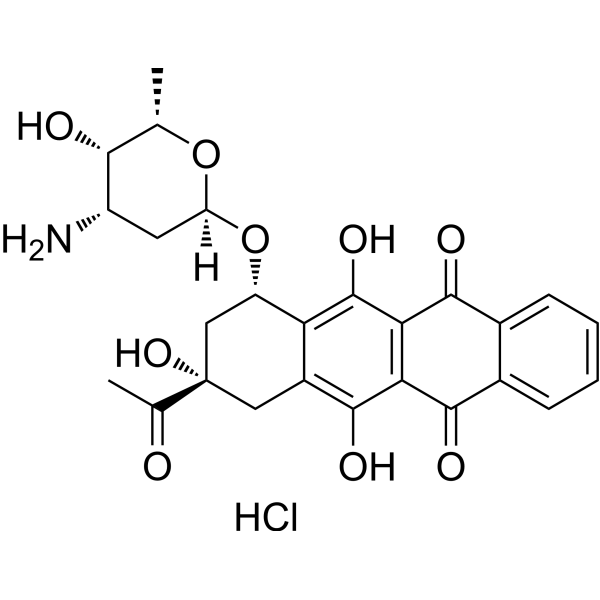
Idarubicin (hydrochloride)
$70 – $400
Products Details
Product Description
– Idarubicin hydrochloride is an anthracycline antileukemic agent. It inhibits the topoisomerase II interfering with the replication of DNA and RNA transcription. Idarubicin hydrochloride inhibits the growth of bacteria and yeasts.
Web ID
– HY-17381
Storage Temperature
– 4°C (Powder, sealed storage, away from moisture and light)
Shipping
– Room Temperature
Applications
– Cancer-programmed cell death
Molecular Formula
– C26H28ClNO9
Citations
– Anal Chem. 2022 Oct 4;94(39):13623-13630.|bioRxiv. 2023 Jan 13.|Cancer Lett. 2019 Oct 1;461:31-43. |Exp Cell Res. 2020 Aug 1;393(1):112054.|J Mol Med (Berl). 2019 Aug;97(8):1183-1193.|J Virol. 2019 May 15;93(11):e02230-18. |Nucleic Acids Res. 2018 Apr 20;46(7):3284-3297.|Viruses. 2020 Jun 10;12(6):628.|Mol Carcinog. 2023 Jul 26.
References
– [1]Robert J. Clinical pharmacokinetics of idarubicin. Clin Pharmacokinet. 1993 Apr;24(4):275-88.|[2]Orlandi P, et al. Idarubicin and idarubicinol effects on breast cancer multicellular spheroids. J Chemother. 2005 Dec;17(6):663-7.|[3]Siegsmund MJ, et al. Enhanced in vitro cytotoxicity of idarubicin compared to epirubicin and doxorubicin in rat prostate carcinoma cells. Eur Urol. 1997;31(3):365-70.|[4]Gewirtz DA, et al. Induction of DNA damage, inhibition of DNA synthesis and suppression of c-myc expression by the anthracycline analog, idarubicin (4-demethoxy-daunorubicin) in the MCF-7 breast tumor cell line. Cancer Chemother Pharmacol. 1998;41(5):361-|[5]Kinnunen U, et al. Idarubicin inhibits the growth of bacteria and yeasts in an automated blood culture system. Eur J Clin Microbiol Infect Dis. 2009 Mar;28(3):301-3.
CAS Number
– 57852-57-0
Molecular Weight
– 533.95
Compound Purity
– 99.94
SMILES
– OC(C(C(C1=CC=CC=C21)=O)=C3C2=O)=C4[C@H](C[C@@](C(C)=O)(O)CC4=C3O)O[C@@](O[C@@H](C)[C@H]5O)([H])C[C@@H]5N.Cl
Clinical Information
– Launched
Research Area
– Cancer; Infection
Solubility
– DMSO : 83.33 mg/mL (ultrasonic)
Target
– Antibiotic;Autophagy;Bacterial;c-Myc;DNA/RNA Synthesis;Fungal;Topoisomerase
Isoform
– Topo II
Pathway
– Anti-infection;Apoptosis;Autophagy;Cell Cycle/DNA Damage
Product type
– Reference compound
Disclaimer: All products are for Research use only unless clearly stated otherwise on the product datasheet. Datasheets provided on the website are drafts for reference purpose only and you are requested to always refer to the hard copy included in the kit for your experimentation. Agdia Products are available for delivery only in Canada.






