Download Files:
Icotinib
SKU
HY-15164A-10 mg
Category Reference compound
Tags Cancer, EGFR, JAK/STAT Signaling;Protein Tyrosine Kinase/RTK
$120 – $670
Products Details
Product Description
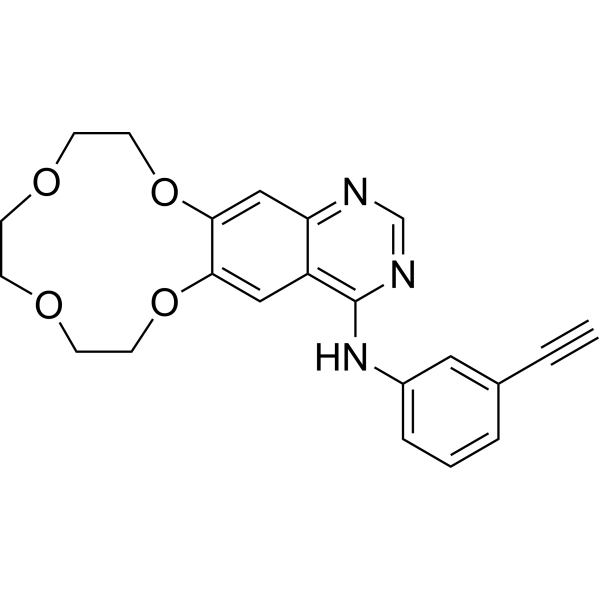
– Icotinib (BPI-2009) is a potent and specific EGFR inhibitor with an IC50 of 5 nM; also inhibits mutant EGFRL858R, EGFRL858R/T790M, EGFRT790M and EGFRL861Q. Icotinib is a click chemistry reagent, it contains an Alkyne group and can undergo copper-catalyzed azide-alkyne cycloaddition (CuAAc) with molecules containing Azide groups.
Web ID
– HY-15164A
Storage Temperature
– -20°C, 3 years (Powder)
Shipping
– Blue Ice
Applications
– Cancer-Kinase/protease
Molecular Formula
– C22H21N3O4
Citations
– Cell Rep Med. 2023 Jan 10;100911.|Clin Chim Acta. 2022 Jan 6;S0009-8981(21)00457-5.|Eur J Pharmacol. 2019 May 5;850:141-149.|J Pharmaceut Biomed. 2023 Jan 26.|Sci Transl Med. 2018 Jul 18;10(450):eaaq1093.|Biochem Pharmacol. 2016 Dec 1;121:67-77. |Science. 2017 Dec 1;358(6367):eaan4368.
References
– [1]Tan F, et al. Icotinib (BPI-2009H), a novel EGFR tyrosine kinase inhibitor, displays potent efficacy in preclinical studies. Lung Cancer. 2012 May;76(2):177-82.
CAS Number
– 610798-31-7
Molecular Weight
– 391.42
Compound Purity
– 99.99
SMILES
– C#CC1=CC(NC2=C(C=C(OCCOCCOCCO3)C3=C4)C4=NC=N2)=CC=C1
Clinical Information
– Launched
Research Area
– Cancer
Solubility
– DMSO : ≥ 155 mg/mL
Target
– EGFR
Isoform
– EGFR/ErbB1/HER1
Pathway
– JAK/STAT Signaling;Protein Tyrosine Kinase/RTK
Product type
– Reference compound
Disclaimer: All products are for Research use only unless clearly stated otherwise on the product datasheet. Datasheets provided on the website are drafts for reference purpose only and you are requested to always refer to the hard copy included in the kit for your experimentation. Agdia Products are available for delivery only in Canada.





