Download Files:
Ibuprofen (L-lysine)
SKU
HY-100586-Get quote
Category Reference compound
Tags Anti-infection;Apoptosis;Immunology/Inflammation, Apoptosis;COX;Parasite, Cancer; Infection; Inflammation/Immunology
Products Details
Product Description
– Ibuprofen ((±)-Ibuprofen) L-lysine is a potent orally active, selective COX-1 inhibitor with an IC50 value of 13 μM. Ibuprofen L-lysine inhibits cell proliferation, angiogenesis, and induces cell apoptosis. Ibuprofen L-lysine is a nonsteroidal anti-inflammatory agent and a nitric oxide (NO) donor. Ibuprofen L-lysine can be used in the research of pain, swelling, inflammation, infection, immunology, cancers[1][2][3][4][5][6][7][8].
Web ID
– HY-100586
Shipping
– Room temperature
Applications
– COVID-19-immunoregulation
Molecular Formula
– C19H32N2O4
References
– [8]M W Konstan, et al. Ibuprofen attenuates the inflammatory response to Pseudomonas aeruginosa in a rat model of chronic pulmonary infection. Implications for antiinflammatory therapy in cystic fibrosis. Am Rev Respir Dis. 1990 Jan;141(1):186-92.|[1]Noreen Y, et al. Development of a radiochemical cyclooxygenase-1 and -2 in vitro assay for identification of natural products as inhibitors of prostaglandin biosynthesis. J Nat Prod. 1998 Jan;61(1):2-7.|[2]Hassan Akrami, et al. Inhibitory effect of ibuprofen on tumor survival and angiogenesis in gastric cancer cell. Tumour Biol. 2015 May;36(5):3237-43.|[3]Sharon M Rymut, et al. Ibuprofen regulation of microtubule dynamics in cystic fibrosis epithelial cells. Am J Physiol Lung Cell Mol Physiol. 2016 Aug 1;311(2):L317-27.|[4]Emmanuelle Bignon, et al. Ibuprofen and ketoprofen potentiate UVA-induced cell death by a photosensitization process. Sci Rep. 2017 Aug 21;7(1):8885.|[5]Nathan D Pennock, et al. Ibuprofen supports macrophage differentiation, T cell recruitment, and tumor suppression in a model of postpartum breast cancer. J Immunother Cancer. 2018 Oct 1;6(1):98.|[6]Thomas Krøigård, et al. Protective effect of ibuprofen in a rat model of chronic oxaliplatin-induced peripheral neuropathy. Exp Brain Res. 2019 Oct;237(10):2645-2651.|[7]Sarah Ilkhanipour Rooney, et al. Ibuprofen Differentially Affects Supraspinatus Muscle and Tendon Adaptations to Exercise in a Rat Model. Am J Sports Med. 2016 Sep;44(9):2237-45.
CAS Number
– 57469-77-9
Molecular Weight
– 352.47
SMILES
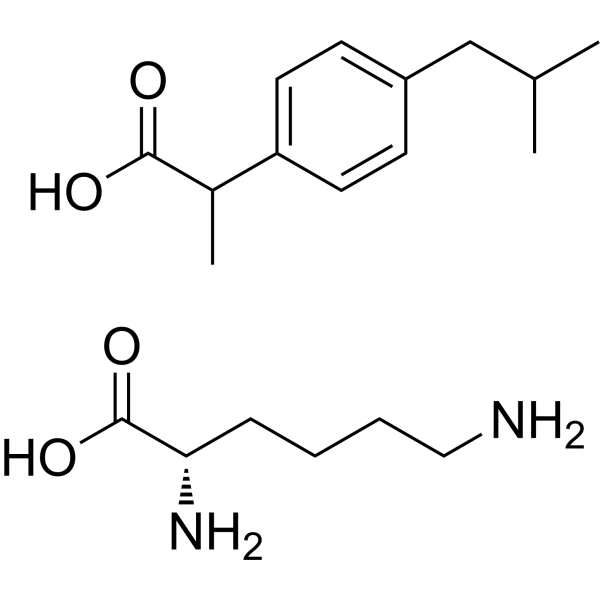
– CC(C(O)=O)C1=CC=C(CC(C)C)C=C1.OC([C@@H](N)CCCCN)=O
Clinical Information
– Launched
Research Area
– Cancer; Infection; Inflammation/Immunology
Solubility
– 10 mM in DMSO
Target
– Apoptosis;COX;Parasite
Pathway
– Anti-infection;Apoptosis;Immunology/Inflammation
Product type
– Reference compound
Disclaimer: All products are for Research use only unless clearly stated otherwise on the product datasheet. Datasheets provided on the website are drafts for reference purpose only and you are requested to always refer to the hard copy included in the kit for your experimentation. Agdia Products are available for delivery only in Canada.






