Download Files:
Ibezapolstat
SKU
HY-128357-10 mg
Category Reference compound
Tags Anti-infection;Cell Cycle/DNA Damage, Bacterial;DNA/RNA Synthesis, Infection
$300 – $2,250
Products Details
Product Description
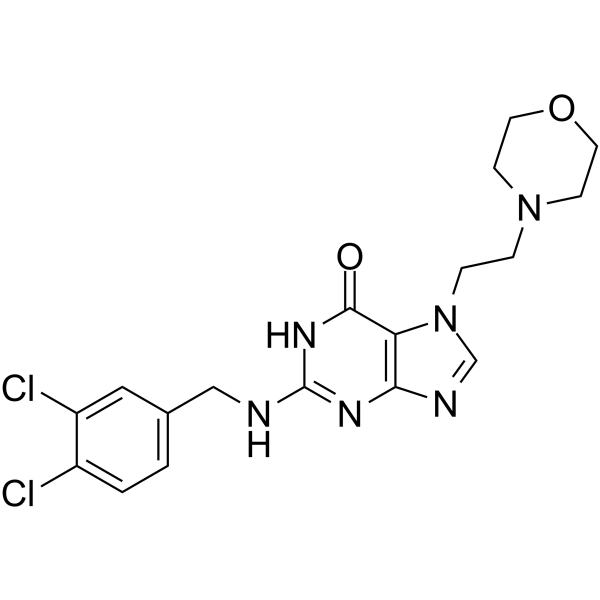
– Ibezapolstat (ACX-362E) is a first-in-class, orally active DNA polymerase IIIC (pol IIIC) inhibitor, with a Ki of 0.325 μM for the DNA pol IIIC from C. difficile. Ibezapolstat is developed for the research of C. difficile infection(CDI)[1][2].
Web ID
– HY-128357
Storage Temperature
– -20°C, 3 years; 4°C, 2 years (Powder)
Shipping
– Room Temperature
Applications
– COVID-19-anti-virus
Molecular Formula
– C18H20Cl2N6O2
References
– [1]Beverly Murray, et al. In vitro activity of the novel antibacterial agent ibezapolstat (ACX-362E) against Clostridioides difficile. J Antimicrob Chemother. 2020 Aug 1;75(8):2149-2155.|[2]Andrea Torti, et al. Clostridium difficile DNA polymerase IIIC: basis for activity of antibacterial compounds. Curr Enzym Inhib. 2011 Oct; 7(3): 147-153.|[3]Sofya Dvoskin, et al. A Novel Agent Effective against Clostridium difficile Infection. Antimicrob Agents Chemother. 2012 Mar; 56(3): 1624-1626.
CAS Number
– 1275582-97-2
Molecular Weight
– 423.30
Compound Purity
– 99.96
SMILES
– O=C1NC(NCC2=CC=C(Cl)C(Cl)=C2)=NC3=C1N(CCN4CCOCC4)C=N3
Clinical Information
– Phase 2
Research Area
– Infection
Solubility
– DMSO : 62.5 mg/mL (ultrasonic)
Target
– Bacterial;DNA/RNA Synthesis
Pathway
– Anti-infection;Cell Cycle/DNA Damage
Product type
– Reference compound
Disclaimer: All products are for Research use only unless clearly stated otherwise on the product datasheet. Datasheets provided on the website are drafts for reference purpose only and you are requested to always refer to the hard copy included in the kit for your experimentation. Agdia Products are available for delivery only in Canada.






