Download Files:
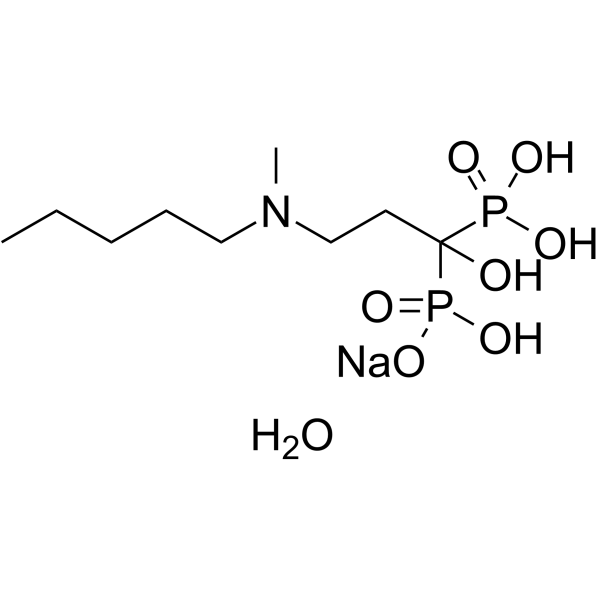
Ibandronate (Sodium Monohydrate)
$144 – $420
Web ID
– HY-B0515
Storage Temperature
– 4°C (Powder, sealed storage, away from moisture)
Shipping
– Room Temperature
Applications
– Cancer-programmed cell death
Molecular Formula
– C9H24NNaO8P2
References
– [1]Morgan, C., S. Jeremiah, and J. Wagstaff, Metronomic administration of ibandronate and its anti-angiogenic effects in vitro. Microvasc Res, 2009. 78(3): p. 453-8.|[2]Epplen, R., et al., Differential effects of ibandronate, docetaxel and farnesol treatment alone and in combination on the growth of prostate cancer cell lines. Acta Oncol, 2011. 50(1): p. 127-33.|[3]Chesnut, I.C., et al., Effects of oral ibandronate administered daily or intermittently on fracture risk in postmenopausal osteoporosis. J Bone Miner Res, 2004. 19(8): p. 1241-9.|[4]Bauss, F., et al., Effects of treatment with ibandronate on bone mass, architecture, biomechanical properties, and bone concentration of ibandronate in ovariectomized aged rats. J Rheumatol, 2002. 29(10): p. 2200-8.
CAS Number
– 138926-19-9
Molecular Weight
– 359.23
Compound Purity
– 98.0
SMILES
– OC(P(O)(O)=O)(P(O)(O[Na])=O)CCN(C)CCCCC.O
Clinical Information
– Launched
Research Area
– Cancer
Solubility
– DMSO : 1 mg/mL (ultrasonic;warming;heat to 80°C)|H2O : 25 mg/mL (ultrasonic)
Target
– Apoptosis
Pathway
– Apoptosis
Product type
– Reference compound
Disclaimer: All products are for Research use only unless clearly stated otherwise on the product datasheet. Datasheets provided on the website are drafts for reference purpose only and you are requested to always refer to the hard copy included in the kit for your experimentation. Agdia Products are available for delivery only in Canada.






