Download Files:
GYKI 53655 (hydrochloride)
SKU
HY-103228-10 mg
Category Reference compound
Tags iGluR, Membrane Transporter/Ion Channel;Neuronal Signaling, Neurological Disease
$120 – $1,200
Products Details
Product Description
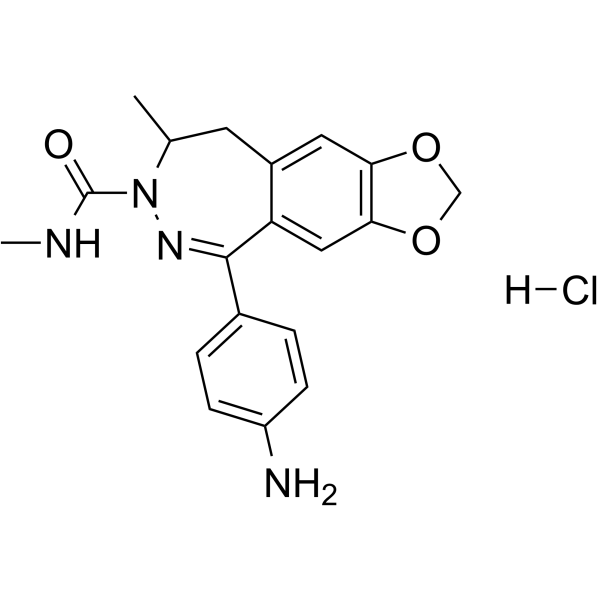
– GYKI 53655 (LY300168) hydrochloride is an α-amino-3-hydroxy-5-methylisoxazole-4-propionic acid (AMPA) antagonist.
Web ID
– HY-103228
Storage Temperature
– 4°C (Powder, sealed storage, away from moisture)
Shipping
– Room Temperature
Applications
– Neuroscience-Neuromodulation
Molecular Formula
– C19H21ClN4O3
References
– [1]Chizh BA, et al. A comparison of intravenous NBQX and GYKI 53655 as AMPA antagonists in the rat spinal cord. Br J Pharmacol. 1994 Jul;112(3):843-6.|[2]Szabados T, et al. Comparison of anticonvulsive and acute neuroprotective activity of three 2,3-benzodiazepine compounds, GYKI 52466, GYKI 53405, and GYKI 53655. Brain Res Bull. 2001 Jun;55(3):387-91.|[3]Bleakman D, et al. Activity of 2,3-benzodiazepines at native rat and recombinant human glutamate receptors in vitro: stereospecificity and selectivity profiles. Neuropharmacology. 1996;35(12):1689-702.
CAS Number
– 143692-48-2
Molecular Weight
– 388.85
Compound Purity
– 98.15
SMILES
– O=C(N1N=C(C2=CC=C(N)C=C2)C3=CC(OCO4)=C4C=C3CC1C)NC.[H]Cl
Clinical Information
– No Development Reported
Research Area
– Neurological Disease
Solubility
– DMSO : ≥ 160 mg/mL|H2O : 8 mg/mL (ultrasonic;warming)
Target
– iGluR
Isoform
– AMPA Receptor
Pathway
– Membrane Transporter/Ion Channel;Neuronal Signaling
Product type
– Reference compound
Disclaimer: All products are for Research use only unless clearly stated otherwise on the product datasheet. Datasheets provided on the website are drafts for reference purpose only and you are requested to always refer to the hard copy included in the kit for your experimentation. Agdia Products are available for delivery only in Canada.





