Download Files:
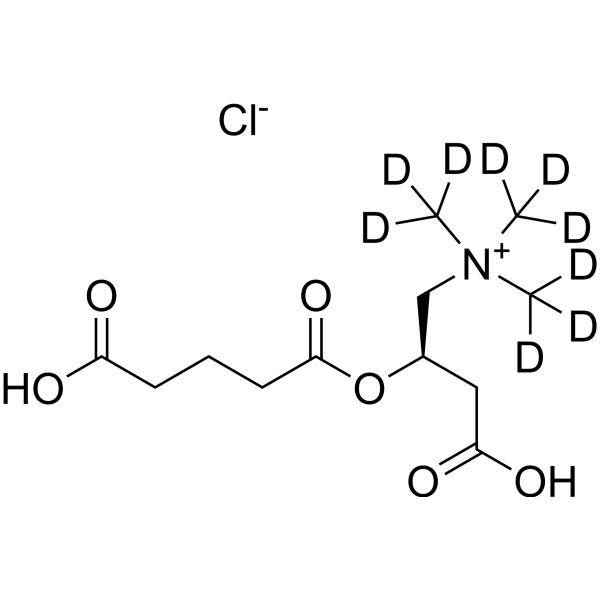
Glutarylcarnitine-d9 (chloride)
SKU
HY-113005S-1 mg
Category Isotope-Labeled Compounds
Tags Endogenous Metabolite;Isotope-Labeled Compounds, Metabolic Disease, Metabolic Enzyme/Protease;Others
$160 – $550
Products Details
Product Description
– Glutarylcarnitine-d9 (chloride) is the deuterium labeled Glutarylcarnitine chloride. Glutarylcarnitine is the diagnostic metabolite for malonic aciduria and glutaric aciduria type I monitored in most tandem mass spectrometry newborn screening programmes.
Web ID
– HY-113005S
Storage Temperature
– 4°C (Powder, sealed storage, away from moisture)
Shipping
– Room Temperature
Molecular Formula
– C12H13D9ClNO6
References
– [1]Russak EM, et al. Impact of Deuterium Substitution on the Pharmacokinetics of Pharmaceuticals. Ann Pharmacother. 2019;53(2):211-216. |[2]Johnson DW, et al. Stability of malonylcarnitine and Glutarylcarnitine in stored blood spots. J Inherit Metab Dis. 2004;27(6):789-90.|[3]S Tortorelli, et al. The urinary excretion of glutarylcarnitine is an informative tool in the biochemical diagnosis of glutaric acidemia type I. Mol Genet Metab. 2005 Feb;84(2):137-43.
Molecular Weight
– 320.81
Compound Purity
– 95.0
SMILES
– O=C(O[C@H](CC(O)=O)C[N+](C([2H])([2H])[2H])(C([2H])([2H])[2H])C([2H])([2H])[2H])CCCC(O)=O.[Cl-]
Clinical Information
– No Development Reported
Research Area
– Metabolic Disease
Solubility
– 10 mM in DMSO
Target
– Endogenous Metabolite;Isotope-Labeled Compounds
Pathway
– Metabolic Enzyme/Protease;Others
Product type
– Isotope-Labeled Compounds
Disclaimer: All products are for Research use only unless clearly stated otherwise on the product datasheet. Datasheets provided on the website are drafts for reference purpose only and you are requested to always refer to the hard copy included in the kit for your experimentation. Agdia Products are available for delivery only in Canada.