Download Files:
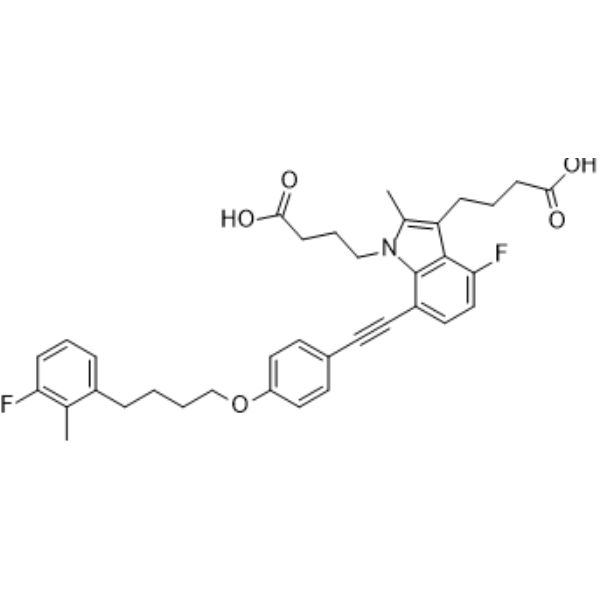
Gemilukast
SKU
HY-16780-10 mg
Category Reference compound
Tags GPCR/G Protein, Inflammation/Immunology, Leukotriene Receptor
$350 – $3,200
Products Details
Product Description
– Gemilukast is an orally active and potent dual cysteinyl leukotriene 1 and 2 receptors (CysLT1 and CysLT2) antagonist, with IC50s of 1.7, 25 nM for human CysLT1 and CysLT2, respectively. Gemilukast is a click chemistry reagent, it contains an Alkyne group and can undergo copper-catalyzed azide-alkyne cycloaddition (CuAAc) with molecules containing Azide groups.
Web ID
– HY-16780
Storage Temperature
– -20°C, 3 years; 4°C, 2 years (Powder)
Shipping
– Room Temperature
Applications
– COVID-19-immunoregulation
Molecular Formula
– C36H37F2NO5
References
– [1]Itadani S, et al. Discovery of Gemilukast (ONO-6950), a Dual CysLT1 and CysLT2 Antagonist As a Therapeutic Agent for Asthma. J Med Chem. 2015 Aug 13;58(15):6093-113.|[2]Yonetomi Y, et al. Effects of ONO-6950, a novel dual cysteinyl leukotriene 1 and 2 receptors antagonist, in a guinea pig model of asthma. Eur J Pharmacol. 2015 Oct 15;765:242-8.
CAS Number
– 1232861-58-3
Molecular Weight
– 601.68
Compound Purity
– 99.50
SMILES
– O=C(O)CCCN1C(C)=C(CCCC(O)=O)C2=C1C(C#CC3=CC=C(OCCCCC4=CC=CC(F)=C4C)C=C3)=CC=C2F
Clinical Information
– No Development Reported
Research Area
– Inflammation/Immunology
Solubility
– DMSO : 250 mg/mL (ultrasonic)
Target
– Leukotriene Receptor
Isoform
– CysLT1;CysLT2
Pathway
– GPCR/G Protein
Product type
– Reference compound
Disclaimer: All products are for Research use only unless clearly stated otherwise on the product datasheet. Datasheets provided on the website are drafts for reference purpose only and you are requested to always refer to the hard copy included in the kit for your experimentation. Agdia Products are available for delivery only in Canada.