Download Files:
Forskolin
$65 – $234
Products Details
Product Description
– Forskolin (Coleonol) is a potent adenylate cyclase activator with an IC50 of 41 nM and an EC50 of 0.5 μM for type I adenylyl cyclase[1]. Forskolin is also an inducer of intracellular cAMP formation[2]. Forskolin induces differentiation of various cell types and activates pregnane X receptor (PXR) and FXR[3]. Forskolin exerts a inotropic effect on the heart, and has platelet antiaggregatory and antihypertensive actions. Forskolin also induces autophagy[4][5].
Web ID
– HY-15371
Storage Temperature
– 4°C (Powder, protect from light)
Shipping
– Room Temperature
Applications
– Cancer-programmed cell death
Molecular Formula
– C22H34O7
References
– [1]Seamon KB, et al. Structure-activity relationships for activation of adenylate cyclase by the diterpene forskolin and its derivatives. J Med Chem. 1983 Mar;26(3):436-9.|[2]Ríos-Silva M, et al. Effect of chronic administration of forskolin on glycemia and oxidative stress in rats with and without experimental diabetes. Int J Med Sci. 2014 Mar 11;11(5):448-52.|[3]Rodriguez G, et al. Forskolin-inducible cAMP pathway negatively regulates T-cell proliferation by uncoupling the interleukin-2 receptor complex. J Biol Chem. 2013 Mar 8;288(10):7137-46.|[4] Amrita Datta, et al. High-throughput screening identified selective inhibitors of exosome biogenesis and secretion: A drug repurposing strategy for advanced cancer. Sci Rep. 2018 May 25;8(1):8161.|[5]Robbins JD, et al. Forskolin carbamates: binding and activation studies with type I adenylyl cyclase. J Med Chem. 1996 Jul 5;39(14):2745-52.|[6]Matsumiya W, et al. Forskolin modifies retinal vascular development in Mrp4-knockout mice. Invest Ophthalmol Vis Sci. 2012 Dec 7;53(13):8029-35. |[7]Mayati A, et al. Functional polarization of human hepatoma HepaRG cells in response to forskolin. Sci Rep. 2018 Oct 31;8(1):16115.|[8]Awad JA, et al. Interactions of forskolin and adenylate cyclase. Effects on substrate kinetics and protection against inactivation by heat and N-ethylmaleimide. J Biol Chem. 1983 Mar 10;258(5):2960-5.
CAS Number
– 66575-29-9
Molecular Weight
– 410.50
Compound Purity
– 99.78
SMILES
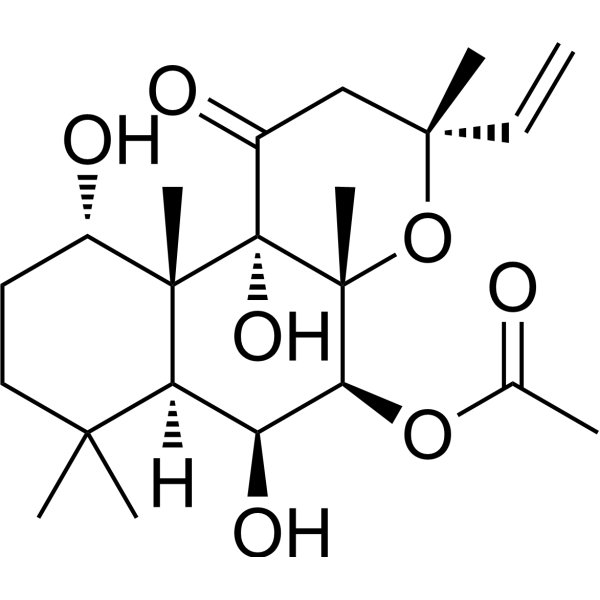
– O=C1[C@@]2(O)[C@]([C@@H](OC(C)=O)[C@@H](O)[C@@]3([H])C(C)(C)CC[C@H](O)[C@@]32C)(C)O[C@@](C)(C=C)C1
Clinical Information
– No Development Reported
Research Area
– Cancer; Endocrinology; Metabolic Disease; Inflammation/Immunology
Solubility
– DMSO : 100 mg/mL (ultrasonic)
Target
– Adenylate Cyclase;Autophagy;FXR;PKC
Pathway
– Autophagy;Epigenetics;GPCR/G Protein;Metabolic Enzyme/Protease;TGF-beta/Smad
Product type
– Natural Products
Disclaimer: All products are for research use only unless clearly stated otherwise on the product datasheet. Datasheets provided on the website are drafts for reference purpose only and you are requested to always refer to the hard copy included in the kit for your experimentation.