Download Files:
Fondaparinux (sodium)
SKU
HY-B0597-1 mg
Category Reference compound
Tags Cardiovascular Disease; Cancer, Factor Xa, Metabolic Enzyme/Protease
$43 – $607
Products Details
Product Description
– Fondaparinux sodium is an antithrombin-dependent factor Xa inhibitor.
Web ID
– HY-B0597
Storage Temperature
– 4°C (Powder, sealed storage, away from moisture)
Shipping
– Room Temperature
Applications
– COVID-19-immunoregulation
Molecular Formula
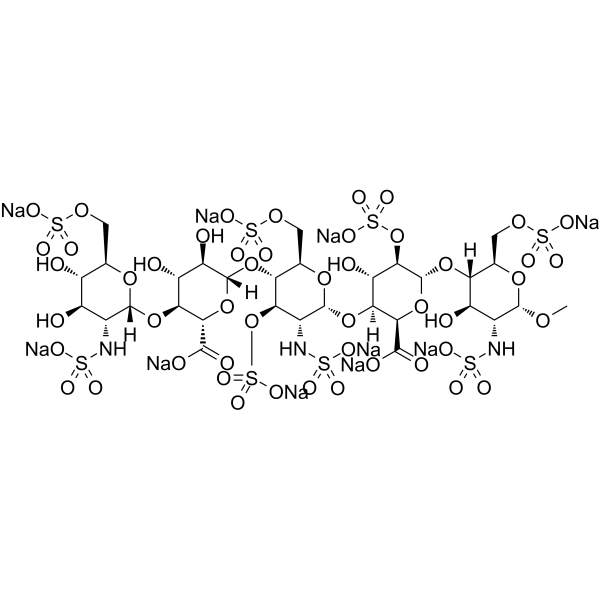
– C31H43N3Na10O49S8
References
– [1]Ben-Hadj-Khalifa S, et al. Differential coagulation inhibitory effect of fondaparinux, enoxaparin and unfractionated heparin in cell models of thrombin generation. Blood Coagul Fibrinolysis. 2011 Jul;22(5):369-73.|[2]Bauer KA. et al. Fondaparinux sodium: a selective inhibitor of factor Xa. Am J Health Syst Pharm. 2001 Nov 1;58 Suppl 2:S14-7.
CAS Number
– 114870-03-0
Molecular Weight
– 1728.08
Compound Purity
– 98.0
SMILES
– O[C@H]([C@@H](O)[C@@H]1O[C@](O[C@H](COS(=O)(O[Na])=O)[C@@H](O)[C@@H]2O)([H])[C@@H]2NS(=O)(O[Na])=O)[C@](O[C@@H]1C(O[Na])=O)([H])O[C@H]([C@H](O[C@@H]3O[C@]([C@@H]4O)([H])[C@@H](O[C@@H](O[C@]([C@@H]5O)([H])[C@H](O[C@H](OC)[C@@H]5NS(=O)(O[Na])=O)COS(=O)(O[Na])=O)[C@@H]4OS(=O)(O[Na])=O)C(O[Na])=O)COS(=O)(O[Na])=O)[C@@H]([C@H]3NS(=O)(O[Na])=O)OS(=O)(O[Na])=O
Clinical Information
– Launched
Research Area
– Cardiovascular Disease; Cancer
Solubility
– H2O : 125 mg/mL (ultrasonic)
Target
– Factor Xa
Pathway
– Metabolic Enzyme/Protease
Product type
– Reference compound
Disclaimer: All products are for Research use only unless clearly stated otherwise on the product datasheet. Datasheets provided on the website are drafts for reference purpose only and you are requested to always refer to the hard copy included in the kit for your experimentation. Agdia Products are available for delivery only in Canada.






