Download Files:
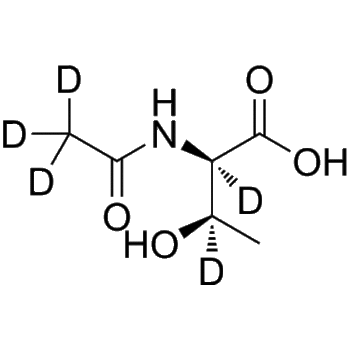
Etoricoxib-d3
Products Details
Product Description
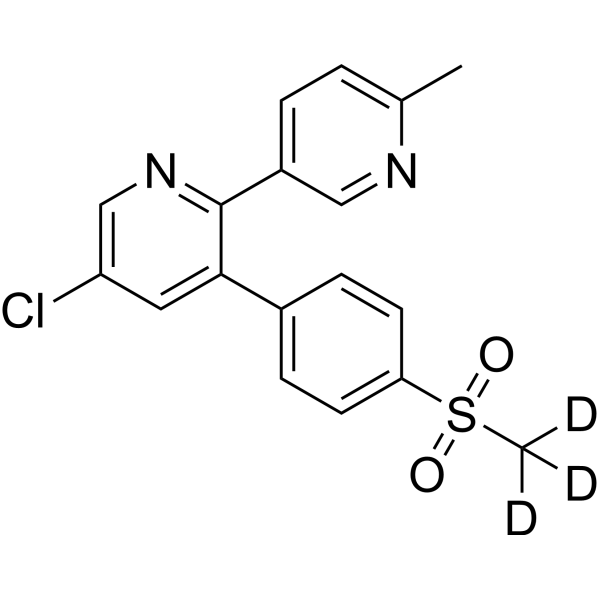
– Etoricoxib-d3 is the deuterium labeled Etoricoxib[1]. Etoricoxib (MK-0663) is a non steroidal anti-inflammatory agent, acting as a selective and orally active COX-2 inhibitor, with IC50s of 1.1 μM and 116 μM for COX-2 and COX-1 in human whole blood[2][3][4].
Web ID
– HY-15321S2
Shipping
– Room temperature
Applications
– COVID-19-immunoregulation
Molecular Formula
– C18H12D3ClN2O2S
References
– [1]Russak EM, et al. Impact of Deuterium Substitution on the Pharmacokinetics of Pharmaceuticals. Ann Pharmacother. 2019 Feb;53(2):211-216.|[2]Riendeau D, et al.Etoricoxib (MK-0663): preclinical profile and comparison with other agents that selectively inhibit cyclooxygenase-2. J Pharmacol Exp Ther. 2001 Feb;296(2):558-66.|[3]Kunak CS, et al. The Effect of Etoricoxib on Hepatic Ischemia-Reperfusion Injury in Rats. Oxid Med Cell Longev. 20152015:598162.|[4]Tanwar L, et al. Anti-proliferative and apoptotic effects of etoricoxib, a selective COX-2 inhibitor, on 1,2-dimethylhydrazine dihydrochloride-induced colon carcinogenesis. Asian Pac J Cancer Prev. 201011(5):1329-33.
CAS Number
– 850896-71-8
Molecular Weight
– 361.86
SMILES
– O=S(C1=CC=C(C2=CC(Cl)=CN=C2C3=CC=C(C)N=C3)C=C1)(C([2H])([2H])[2H])=O
Clinical Information
– No Development Reported
Solubility
– 10 mM in DMSO
Target
– COX
Pathway
– Immunology/Inflammation
Product type
– Isotope-Labeled Compounds
Disclaimer: All products are for Research use only unless clearly stated otherwise on the product datasheet. Datasheets provided on the website are drafts for reference purpose only and you are requested to always refer to the hard copy included in the kit for your experimentation. Agdia Products are available for delivery only in Canada.