Download Files:
Epitinib
SKU
HY-139300-Get quote
Category Reference compound
Tags Cancer, EGFR, JAK/STAT Signaling;Protein Tyrosine Kinase/RTK
Products Details
Product Description
– Epitinib is an orally active and selective epidermal growth factor receptor tyrosine kinase inhibitor (EGFR-TKI) designed for optimal brain penetration. Epitinib can be used for the research of cancer[1][2]. Epitinib is a click chemistry reagent, it contains an Alkyne group and can undergo copper-catalyzed azide-alkyne cycloaddition (CuAAc) with molecules containing Azide groups.
Web ID
– HY-139300
Shipping
– Room temperature
Applications
– Cancer-Kinase/protease
Molecular Formula
– C24H26N6O2
References
– [1] Zhou et al. The safety profile of a selective EGFR TKI epitinib (HMPL-813) in patients with advanced solid tumors and preliminary clinical efficacy in EGFRm+ NSCLC patients with brain metastasis. Journal of Clinical Oncology 2016 34:15_suppl, e20502-e20502|[2] Zhou et al. Phase I study of the safety and pharmacokinetics of epitinib, an oral EGFR tyrosine kinase inhibitor, in patients with advanced solid tumors. Journal of Clinical Oncology 2013 31:15_suppl, e19042-e19042
CAS Number
– 1203902-67-3
Molecular Weight
– 430.50
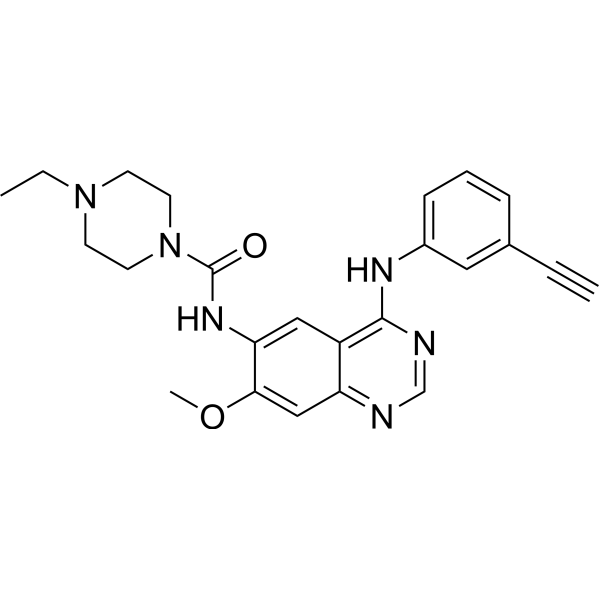
SMILES
– O=C(N1CCN(CC)CC1)NC2=CC3=C(NC4=CC=CC(C#C)=C4)N=CN=C3C=C2OC
Clinical Information
– Phase 1
Research Area
– Cancer
Solubility
– 10 mM in DMSO
Target
– EGFR
Pathway
– JAK/STAT Signaling;Protein Tyrosine Kinase/RTK
Product type
– Reference compound
Disclaimer: All products are for research use only unless clearly stated otherwise on the product datasheet. Datasheets provided on the website are drafts for reference purpose only and you are requested to always refer to the hard copy included in the kit for your experimentation.






