Download Files:
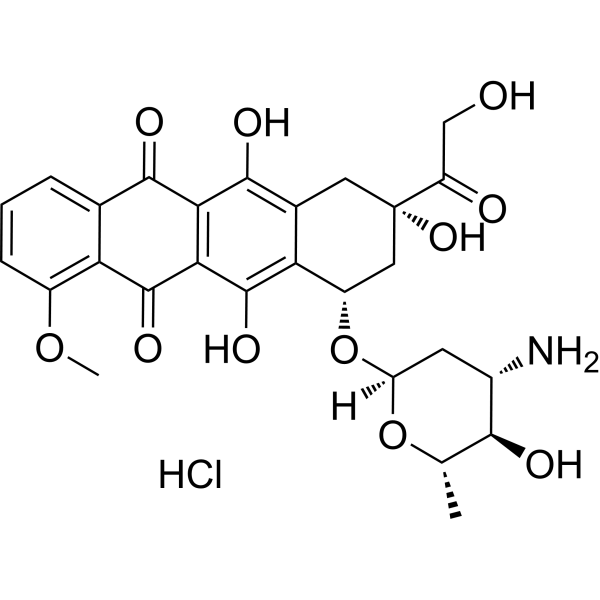
Epirubicin (hydrochloride)
SKU
HY-13624A-10 mg
Category Reference compound
Tags Anti-infection;Apoptosis;Cell Cycle/DNA Damage, Antibiotic;Apoptosis;DNA/RNA Synthesis;Topoisomerase, Cancer
$66 – $422
Products Details
Product Description
– Epirubicin hydrochloride (4′-Epidoxorubicin hydrochloride), a semisynthetic L-arabino derivative of doxorubicin, has an antineoplastic agent by inhibiting Topoisomerase[1]. Epirubicin hydrochloride inhibits DNA and RNA synthesis. Epirubicin hydrochloride is a Forkhead box protein p3 (Foxp3) inhibitor and inhibits regulatory T cell activity[2].
Web ID
– HY-13624A
Storage Temperature
– 4°C (Powder, sealed storage, away from moisture and light)
Shipping
– Room Temperature
Applications
– Cancer-programmed cell death
Molecular Formula
– C27H30ClNO11
Citations
– Anal Chem. 2022 Oct 4;94(39):13623-13630.|bioRxiv. 2023 Jan 13.|Cancer Res Treat. 2023 Jun 12.|Cell Mol Immunol. 2023 Jan;20(1):51-64.|J Mol Med (Berl). 2019 Aug;97(8):1183-1193.|Mol Cell. 2022 Feb 17;S1097-2765(22)00085-5.|Research Square Preprint. 2021 Nov.|Asia Pac J Clin Oncol. 2022 Dec 4.|bioRxiv. 2023 Apr 17.|Cell Prolif. 2021 Apr 1;e13038.|Exp Cell Res. 2020 Aug 1;393(1):112054.|Genomics. 2021 Nov 26;S0888-7543(21)00410-9.|Int J Mol Sci. 2023, 24(1), 343.|J Biomed Inform. 2023 May 15;104383.|Mol Pharm. 2019 Aug 5;16(8):3452-3459. |Oncol Lett. 2020 Apr. |Research Square Preprint. 2020 May.|Yonsei Med J. 2019 Sep;60(9):832-841.
References
– [1]Kashima H, et al. Epirubicin, Identified Using a Novel Luciferase Reporter Assay for Foxp3 Inhibitors, Inhibits Regulatory T Cell Activity. PLoS One. 2016 Jun 10;11(6):e0156643.|[2]Bonadonna, G., et al. Drugs ten years later: epirubicin. Ann Oncol, 1993. 4(5): p. 359-69.|[3]Asanuma, F., et al. Antitumor activity of paclitaxel and epirubicin in human breast carcinoma, R-27. Folia Microbiol (Praha), 1998. 43(5): p. 473-4.|[4]Ozkan, A., et al. Epirubicin HCl toxicity in human-liver derived hepatoma G2 cells. Pol J Pharmacol, 2004. 56(4): p. 435-44.|[5]Cersosimo RJ, et al. Epirubicin: a review of the pharmacology, clinical activity, and adverse effects of an adriamycin analogue. J Clin Oncol. 1986 Mar;4(3):425-39.
CAS Number
– 56390-09-1
Molecular Weight
– 579.98
Compound Purity
– 99.16
SMILES
– O=C(C1=C2C(O)=C3[C@@H](O[C@@]4([H])C[C@H](N)[C@@H](O)[C@H](C)O4)C[C@@](C(CO)=O)(O)CC3=C1O)C5=CC=CC(OC)=C5C2=O.Cl
Clinical Information
– Launched
Research Area
– Cancer
Solubility
– DMSO : 25 mg/mL (ultrasonic)|H2O : 50 mg/mL (ultrasonic)
Target
– Antibiotic;Apoptosis;DNA/RNA Synthesis;Topoisomerase
Isoform
– Topoisomerase
Pathway
– Anti-infection;Apoptosis;Cell Cycle/DNA Damage
Product type
– Reference compound
Disclaimer: All products are for Research use only unless clearly stated otherwise on the product datasheet. Datasheets provided on the website are drafts for reference purpose only and you are requested to always refer to the hard copy included in the kit for your experimentation. Agdia Products are available for delivery only in Canada.