Download Files:
Enoxacin (hydrate)
SKU
HY-B0268A-100 mg
Category Reference compound
Tags Anti-infection;Cell Cycle/DNA Damage;Epigenetics, Antibiotic;Bacterial;DNA/RNA Synthesis;MicroRNA, Infection; Cancer
$60 – $96
Products Details
Product Description
– Enoxacin hydrate (Enoxacin sesquihydrate), a fluoroquinolone, interferes with DNA replication and inhibits bacterial DNA gyrase (IC50=126 µg/ml) and topoisomerase IV (IC50=26.5 µg/ml). Enoxacin hydrate is a miRNA processing activator and enhances siRNA-mediated mRNA degradation and promotes the biogenesis of endogenous miRNAs. Enoxacin hydrate has potent activities against gram-positive and -negative bacteria. Enoxacin hydrate is a cancer-specific growth inhibitor that acts by enhancing TAR RNA-binding protein 2 (TRBP)-mediated microRNA processing[1][2][3][4].
Web ID
– HY-B0268A
Storage Temperature
– 4°C (Powder, sealed storage, away from moisture and light)
Shipping
– Room Temperature
Applications
– COVID-19-immunoregulation
Molecular Formula
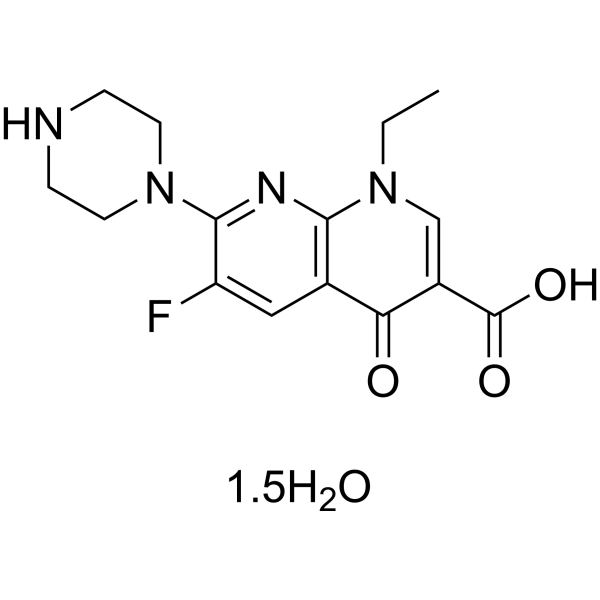
– C15H17FN4O3.3/2H2O
References
– [1]Rengen Fan, et al. Small molecules with big roles in microRNA chemical biology and microRNA-targeted therapeutics. RNA Biol. 2019 Jun;16(6):707-718.|[2]Ge Shan, et al. A small molecule enhances RNA interference and promotes microRNA processing. Nat Biotechnol. 2008 Aug;26(8):933-40.|[3]Sonia Melo, et al.Small molecule enoxacin is a cancer-specific growth inhibitor that acts by enhancing TAR RNA-binding protein 2-mediated microRNA processing. Proc Natl Acad Sci U S A. 2011 Mar 15;108(11):4394-9.|[4]M Takei, et al. Target preference of 15 quinolones against Staphylococcus aureus, based on antibacterial activities and target inhibition. Antimicrob Agents Chemother. 2001 Dec;45(12):3544-7.|[5]Chin, N.-X. and H.C. Neu, In vitro activity of enoxacin, a quinolone carboxylic acid, compared with those of norfloxacin, new beta-lactams, aminoglycosides, and trimethoprim. Antimicrobial agents and chemotherapy, 1983. 24(5): p. 754-763.
CAS Number
– 84294-96-2
Molecular Weight
– 347.34
Compound Purity
– 99.85
SMILES
– O=C(C1=CN(CC)C2=C(C=C(F)C(N3CCNCC3)=N2)C1=O)O.[1.5H2O]
Clinical Information
– Launched
Research Area
– Infection; Cancer
Solubility
– 1M NaOH : 100 mg/mL (ultrasonic;adjust pH to 11 with NaOH)|DMSO : 2.78 mg/mL (ultrasonic)|H2O : 1 mg/mL (ultrasonic;warming;heat to 80°C)
Target
– Antibiotic;Bacterial;DNA/RNA Synthesis;MicroRNA
Isoform
– Quinolone
Pathway
– Anti-infection;Cell Cycle/DNA Damage;Epigenetics
Product type
– Reference compound
Disclaimer: All products are for Research use only unless clearly stated otherwise on the product datasheet. Datasheets provided on the website are drafts for reference purpose only and you are requested to always refer to the hard copy included in the kit for your experimentation. Agdia Products are available for delivery only in Canada.






