Download Files:
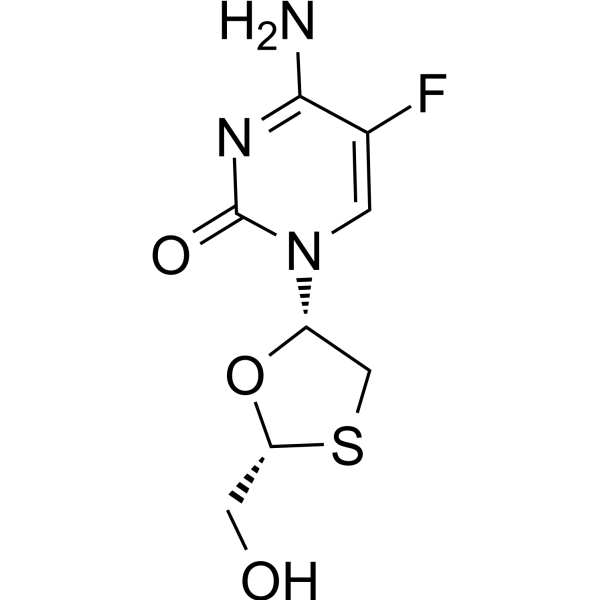
Emtricitabine
SKU
HY-17427-100 mg
Category Reference compound
Tags Anti-infection;Metabolic Enzyme/Protease, Endogenous Metabolite;HIV;Reverse Transcriptase, Infection
$55 – $149
Products Details
Product Description
– Emtricitabine is a nucleoside reverse transcriptase inhibitor (NRTI) with an EC50 of 0.01 μM in PBMC cell. It is an antiviral agent for the treatment of HIV infection.
Web ID
– HY-17427
Storage Temperature
– -20°C, 3 years; 4°C, 2 years (Powder)
Shipping
– Room Temperature
Applications
– COVID-19-anti-virus
Molecular Formula
– C8H10FN3O3S
References
– [1]Xu P, et al. Combined Medication of Antiretroviral Drugs Tenofovir Disoproxil Fumarate, Emtricitabine, and Raltegravir Reduces Neural Progenitor Cell Proliferation In Vivo and In Vitro. J Neuroimmune Pharmacol. 2017 Dec;12(4):682-692.|[2]Faltz M, et al. Effect of the Anti-retroviral Drugs Efavirenz, Tenofovir and Emtricitabine on Endothelial Cell Function: Role of PARP. Cardiovasc Toxicol. 2017 Jan 3. [Epub ahead of print]|[3]Szczech GM, Wang LH, Walsh JP, Reproductive toxicology profile of emtricitabine in mice and rabbits. Reprod Toxicol. 2003 Jan-Feb;17(1):95-108.|[4]Saag MS, et al. Emtricitabine, a new antiretroviral agent with activity against HIV and hepatitis B virus. Clin Infect Dis.?2006 Jan 1;42(1):126-31.
CAS Number
– 143491-57-0
Molecular Weight
– 247.25
Compound Purity
– 99.94
SMILES
– O=C1N=C(N)C(F)=CN1[C@@H]2CS[C@H](CO)O2
Clinical Information
– Launched
Research Area
– Infection
Solubility
– DMSO : ≥ 100 mg/mL|H2O : ≥ 25 mg/mL
Target
– Endogenous Metabolite;HIV;Reverse Transcriptase
Isoform
– HIV-1;HIV-2
Pathway
– Anti-infection;Metabolic Enzyme/Protease
Product type
– Reference compound
Disclaimer: All products are for Research use only unless clearly stated otherwise on the product datasheet. Datasheets provided on the website are drafts for reference purpose only and you are requested to always refer to the hard copy included in the kit for your experimentation. Agdia Products are available for delivery only in Canada.






