Download Files:
Elagolix sodium
SKU
HY-14369-10 mg
Category Reference compound
Tags Endocrinology; Cancer, GnRH Receptor, GPCR/G Protein
$60 – $460
Products Details
Product Description
– Elagolix sodium is a human GnRH receptor (GnRHR) antagonist with an IC50 and Ki of 0.25 and 3.7 nM, respectively.
Web ID
– HY-14369
Storage Temperature
– 4°C (Powder, sealed storage, away from moisture)
Shipping
– Room Temperature
Applications
– Cancer-Kinase/protease
Molecular Formula
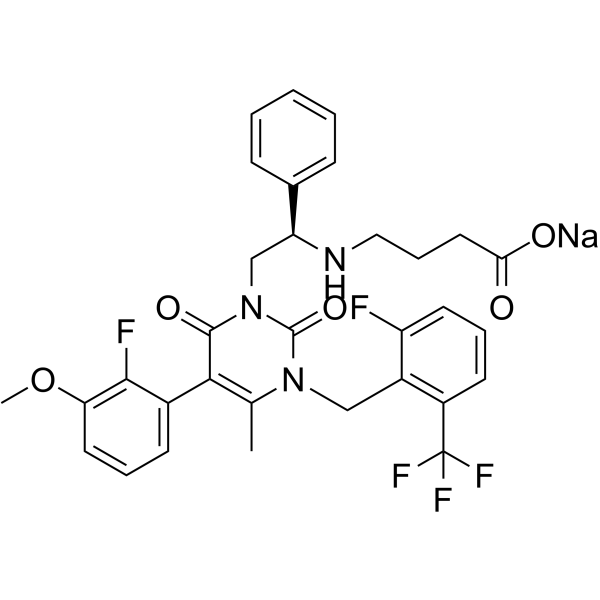
– C32H29F5N3NaO5
References
– [1]Kim SM, et al. Discovery of an Orally Bioavailable Gonadotropin-Releasing Hormone Receptor Antagonist. J Med Chem. 2016 Oct 13;59(19):9150-9172. Epub 2016 Sep 27.|[2]Chen C, et al. Discovery of sodium R-(+)-4-{2-[5-(2-fluoro-3-methoxyphenyl)-3-(2-fluoro-6-[trifluoromethyl]benzyl)-4-methyl-2,6-dioxo-3,6-dihydro-2H-pyrimidin-1-yl]-1-phenylethylamino}butyrate (elagolix), a potent and orally available nonpeptide antagonist of the human gonadotropin-releasing hormone receptor. J Med Chem. 2008 Dec 11;51(23):7478-85.
CAS Number
– 832720-36-2
Molecular Weight
– 653.57
Compound Purity
– 99.66
SMILES
– O=C(CCCN[C@@H](CN(C1=O)C(N(C(C)=C1C2=CC=CC(OC)=C2F)CC3=C(C=CC=C3F)C(F)(F)F)=O)C4=CC=CC=C4)O[Na]
Clinical Information
– Launched
Research Area
– Endocrinology; Cancer
Solubility
– DMSO : 50 mg/mL (ultrasonic)|H2O : ≥ 50 mg/mL
Target
– GnRH Receptor
Pathway
– GPCR/G Protein
Product type
– Reference compound
Disclaimer: All products are for Research use only unless clearly stated otherwise on the product datasheet. Datasheets provided on the website are drafts for reference purpose only and you are requested to always refer to the hard copy included in the kit for your experimentation. Agdia Products are available for delivery only in Canada.






