Download Files:
Edoxaban impurity 4
SKU
HY-134686-10 mg
Category Reference compound
Tags Cardiovascular Disease, Factor Xa;Thrombin, Metabolic Enzyme/Protease
$220 – $350
Products Details
Product Description
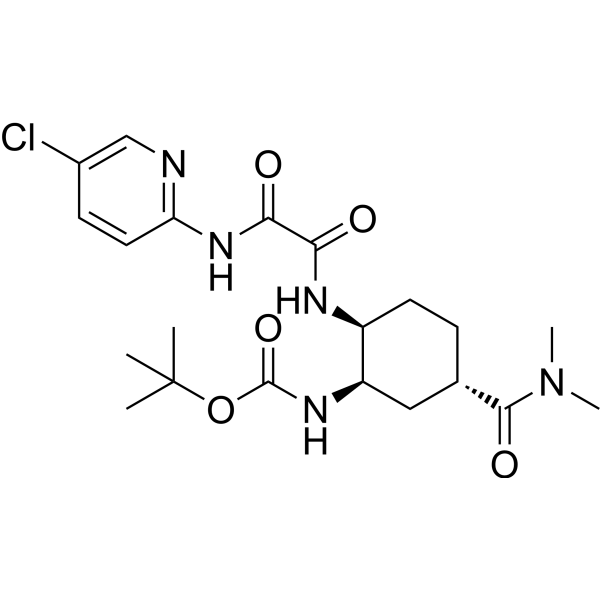
– Edoxaban impurity 4 is an impurity of Edoxaban. Edoxaban (DU-176) is a selective, potent and orally active factor Xa (FXa) inhibitor with Kis of 0.561 nM and 2.98 nM for free FXa and prothrombinase, respectively. Edoxaban is an anticoagulant agent and can be used for stroke prevention[1][2].
Web ID
– HY-134686
Storage Temperature
– 4°C (Powder, protect from light)
Shipping
– Room Temperature
Applications
– Neuroscience-Neuromodulation
Molecular Formula
– C21H30ClN5O5
References
– [1]Furugohri T, et al. DU-176b, a potent and orally active factor Xa inhibitor: in vitro and in vivo pharmacological profiles. J Thromb Haemost. 2008 Sep;6(9):1542-9.|[2]Mendell J, Lee F, Chen S, The Effects of the Antiplatelet Agents, Aspirin and Naproxen, on Pharmacokinetics and Pharmacodynamics of the Anticoagulant Edoxaban, a Direct Factor Xa Inhibitor. J Cardiovasc Pharmacol. 2013 Apr 23.
CAS Number
– 480452-36-6
Molecular Weight
– 467.95
Compound Purity
– 98.92
SMILES
– O=C(OC(C)(C)C)N[C@H]1[C@@H](NC(C(NC2=NC=C(Cl)C=C2)=O)=O)CC[C@H](C(N(C)C)=O)C1
Clinical Information
– No Development Reported
Research Area
– Cardiovascular Disease
Solubility
– DMSO : 50 mg/mL (ultrasonic)
Target
– Factor Xa;Thrombin
Pathway
– Metabolic Enzyme/Protease
Product type
– Reference compound
Disclaimer: All products are for Research use only unless clearly stated otherwise on the product datasheet. Datasheets provided on the website are drafts for reference purpose only and you are requested to always refer to the hard copy included in the kit for your experimentation. Agdia Products are available for delivery only in Canada.





