Download Files:
DUPA(OtBu)-OH
$180 – $910
Products Details
Product Description
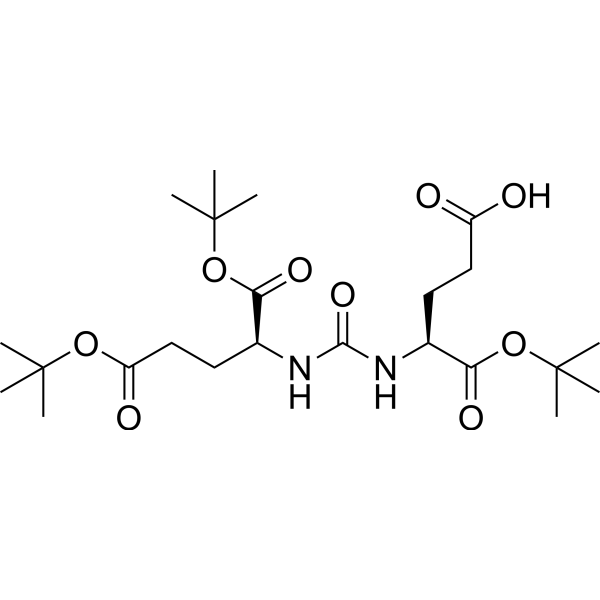
– DUPA(OtBu)-OH is a DUPA precursor. DUPA is used as the targeting moiety to actively deliver Docetaxel (DTX) for treatment of Prostate-Specific Membrane Antigen (PSMA) expressing prostate cancer.
Web ID
– HY-103591
Storage Temperature
– -20°C, 3 years; 4°C, 2 years (Powder)
Shipping
– Room Temperature
Applications
– Cancer-programmed cell death
Molecular Formula
– C23H40N2O9
References
– [1]Roy J, et al. DUPA conjugation of a cytotoxic indenoisoquinoline topoisomerase I inhibitor for selectiveprostate cancer cell targeting. J Med Chem. 2015 Apr 9;58(7):3094-103.|[2]Lv Q, et al. Prostate-Specific Membrane Antigen Targeted Therapy of Prostate Cancer Using a DUPA-Paclitaxel Conjugate. Mol Pharm. 2018 May 7;15(5):1842-1852.|[3]Peng ZH, et al. Spacer length impacts the efficacy of targeted docetaxel conjugates in prostate-specificmembrane antigen expressing prostate cancer. J Drug Target. 2013 Dec;21(10):968-80.
CAS Number
– 1026987-94-9
Molecular Weight
– 488.57
Compound Purity
– 98.0
SMILES
– O=C(OC(C)(C)C)CC[C@@H](C(OC(C)(C)C)=O)NC(N[C@H](C(OC(C)(C)C)=O)CCC(O)=O)=O
Clinical Information
– No Development Reported
Research Area
– Cancer
Solubility
– DMSO : 125 mg/mL (ultrasonic)
Target
– PSMA
Pathway
– Immunology/Inflammation
Product type
– Reference compound
Disclaimer: All products are for Research use only unless clearly stated otherwise on the product datasheet. Datasheets provided on the website are drafts for reference purpose only and you are requested to always refer to the hard copy included in the kit for your experimentation. Agdia Products are available for delivery only in Canada.






